Q1: A liquid mixture of ethanol and water is flowing as inlet stream P into a stream splitter. It is split into two streams, Q and R, as shown in the figure below.
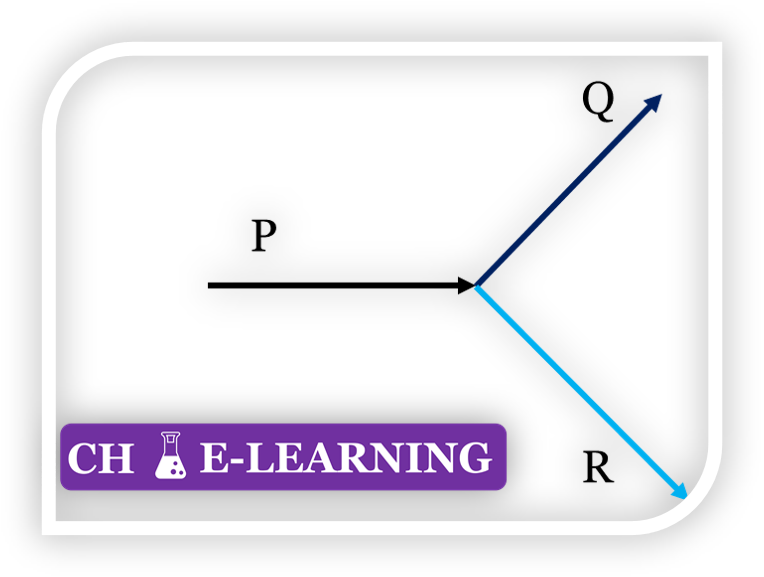
The flow rate of P, containing 30 mass% of ethanol, is 100 kg/h. What is the least number of additional specification(s) required to determine the mass flow rates and compositions (mass%) of the two exit streams?
Q 2: A jacketed stirred tank with a provision for heat removal is used to mix sulphuric acid and water in a steady-state flow process. H2SO4 (l) enters at a rate of 4 kg/h at 25 0C and H2O (l) enters at a rate of 6 kg/h at 10 0C. The following data are available:
Specific heat capacity of water = 4.2 kJ kg‒1K‒1. Specific heat capacity of the aqueous solution of 40 mass% H2SO4 = 2.8 kJ (kg solution)‒1 K‒1. Assume the specific heat capacities to be independent of temperature. Based on reference states of H2SO4 (l) and H2O (l) at 25 0C, the heat of mixing for an aqueous solution of 40 mass% H2SO4 = ‒ 650 kJ (kg H2SO4)‒1.
If the mixed stream leaves at 40 0C, what is the rate of heat removal (in kJ/h)?
Q 3: A catalytic reforming plant produces hydrogen and benzene from cyclohexane by de-hydro aromatization. In order to increase the production of hydrogen, the owner plans to change the process to steam reforming of the same feedstock that produces hydrogen and carbon dioxide. Stoichiometrically, what is the maximum ratio of pure hydrogen produced in the proposed process to that in the existing process?
