Problem 9.1: Wet sewage sludge enters a continuous thickener at a rate of 100 kg per hour and dehydrated sludge leaves the thickener at a rate of 75 kg per hour. Determine the amount of water removed in the thickener in one hour, assuming steady-state operation.
Problem 9.2: Air is dehumidified at a constant pressure of 101.3 kPa. The partial pressure of water in the air admitted to the dehumidifier is 7 kPa and the temperature is 350 K. The partial pressure of water in the air leaving is 1.5 kPa. How much water (in kilograms) is removed from 100 cubic meters of air admitted?
Problem 9.3: Urea, phosphoric acid, and potassium chloride are mixed together to obtain a mixed fertilizer having NPK content 10: 26: 26 as %N, %P2O5, and %K2O by weight, the balance being the weight of filler materials. Calculate the quantities to be mixed to get 1000 kg of mixed fertilizer.
Problem 9.4: Formulate the independent material balance equations for determining the flow rate of benzene in the feed under the following circumstances. An aqueous acetic acid solution containing 80 % acetic acid and the rest water is charged into a still along with pure benzene. 400 kg/h pure acetic acid is withdrawn as a product from the still. The top product leaving the still consists of 11.0 % acetic acid, 21.5 % water, and the rest benzene.
Problem 9.5: A soap plant produces raw soap containing 50 % moisture. This is to be dried to 20 % moisture before it is pressed into cakes for sale. How many 100-g soap pieces can be obtained from 1000 kg of original raw soap?
Problem 9.6: A weak acid containing 12.5% H2SO4 and the rest water is fortified by adding 500 kg of concentrated acid containing 80 % H2SO4. Determine the amount of the solution obtained if it contains 18.5% H2SO4.
Problem 9.7: A cellulose solution contains 5.0 % cellulose by weight in water. It is to be diluted to 4.0 % using a 1.0 % solution of cellulose in water. Determine the kilograms of 1.0 % solution required to dilute 100 kg of the 5.0 % solution.
Problem 9.8: One hundred kilograms of dilute waste acid containing 30.0 % sulphuric acid is to be fortified to 50.0 % sulphuric acid using concentrated sulphuric acid of strength 96.0 %. How many kilograms of concentrated sulphuric acid are required for this process?
Problem 9.9: Waste acid from a nitrating process contains 25 % HNO3, 55 % H2SO4, and 20 % H2O by weight. This is to be concentrated to get fortified acid containing 27 % HNO3, 60 % H2SO4, and 13 % water. This is done by adding concentrated H2SO4 of strength 93 % H2SO4 and concentrated HNO3 of strength 90 % HNO3 in suitable quantities to the waste acid. If 1000 kg fortified acid is to be produced, calculate the kg of the various solutions mixed.
Problem 9.10: The liquid effluent from a processing plant having a BOD of 0.15 g/L is discharged at a rate of 4000 cubic meters per day into a stream flowing at a rate of 0.3 cubic meters per second and having an initial BOD of 5×10-3 g/L. Determine the BOD in the stream immediately below the discharge point.
Problem 9.11: It is decided to measure the flow rate of a pure air stream by injecting pure CO2 at a rate of 10 mol/h into the flowing stream. The resultant mixture analyzed 8.6 % CO2 on a mole basis. What is the flow rate of air?
Problem 9.12: Pure oxygen at 120 kPa and 300K is injected into an ammonia pipeline at a rate of 0.02 m3/s. The concentration of oxygen in the pipeline at a point far removed from the point of injection is found to be 10 % (volume). What is the flow rate of ammonia through the pipe in kg/h?
Problem 9.13: Two tanks that are connected to each other are initially sealed off from one another by means of a valve. Tank I initially contained 1 m3 of air at 600 kPa and 343.2 K. Tank II contained a mixture of oxygen and nitrogen containing 95 % (mole) nitrogen at 1200 kPa and 363.2 K. The valve is now opened and the contents of the tanks are allowed to mix. After complete mixing, the tanks contained 85 % (mole) nitrogen. Calculate the volume of tank II.
Problem 9.14: It is desired to produce a gas mixture analyzing 40 % methane, 35 % ethane, and 25 % propane by blending the following three gas mixtures in suitable proportions.
| Constituent | Mixture I | Mixture II | Mixture III |
| CH4 | 25 | 35 | 55 |
| C2H6 | 35 | 20 | 40 |
| C3H8 | 40 | 45 | 5 |
Determine the proportion in which the gases are to be mixed.
Problem 9.15: A process stream of a given flow rate is obtained by mixing together two separate streams. In order to determine the ratio in which the streams are mixed, a soluble salt is added to one of the streams at a steady rate. The concentration of salt in the stream is determined to be 5 % (W) and that in the combined stream is found to be 0.45 % (W). Determine the ratio in which the streams are mixed.
Problem 9.16: A laundry can purchase soap containing 30 % by weight water at a rate of Rs14 per kg f.o.b. factory. The same manufacturer offers a soap containing 5 % water by weight. If the freight rate is Rs 1.40 per 10 kg, what is the maximum price that the laundry can pay the manufacturer for the soap containing 5 % water?
Problem 9.17: Rubber latex containing 15 % (weight) rubber solids is to be coagulated by treating with 66°Bé H2SO4. If the ratio of the weights of acid to latex solids is 1:50, determine the following: (a) Cubic meters/hour of 66°Bé acid required for treating 1000 kg/h of latex (b) How many kilograms of solution to be removed per hour for recovering the solids.
Problem 9.18: A company has drawn a contract for the purchase of paper at a price of Rs 10 per kg of paper containing 5 % moisture by weight and to adjust the price if the moisture content varies, so that the price of bone-dry paper is constant. Also, if the moisture content exceeds 5 %, the additional freight charge incurred due to the excess moisture will be deducted from the total cost. The freight charge is Rs 2 per kg of paper. Calculate the following: (a) The total amount to be paid for 10000 kg of paper containing 3 % moisture (b) The total amount to be paid for 8000 kg of paper containing 9 % moisture.
Problem 9.19: A water sample taken from a stream contained 200 ppm Na2SO4. To measure the flow rate of the running stream, 5 kg Na2SO4 is added to the stream uniformly over a one- hour period. The analysis of water taken from the stream from a spot downstream from the point of addition of the salt showed 350 ppm Na2SO4. What is the rate of flow of water in the stream?
Problem 9.20: The ion exchange process shown in Figure below is used for water purification.
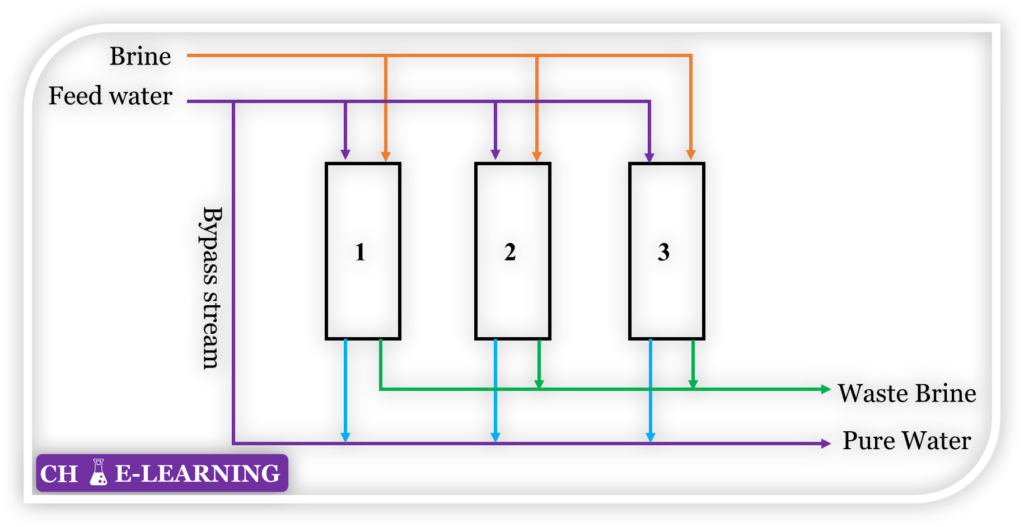
Here, cations such as Ca++ and Mg++ are exchanged with Na+ ions in the ion-exchange resin, resins are subsequently regenerated with concentrated brine. The process data for the unit is given below:
| Feedwater (ppm) | Tolerable limit in pure water (ppm) | Fresh brine (ppm) | Waste brine (ppm) | |
| Ca++ | 400 | 10 | 500 | 41500 |
| Mg++ | 250 | 15 | 300 | 25000 |
| Na+ | 60 | 800 | ~2×105 | ~2×105 |
At any given time when one bed purifies hard water, one is engaged in the regeneration of the used-up bed and the third one is kept idle as a standby unit. Determine the average brine consumption per 1000 kg of process water being treated.
Problem 9.21: In a process for producing caustic (NaOH), 4000 kg/h of a solution containing 10 wt% NaOH is evaporated in the first evaporator, giving a 20 % NaOH solution. This is then fed into a second evaporator, which gives a product of 50 % NaOH. Calculate the following: (a) The amount of water removed from each evaporator (b) The feed to the second evaporator, kg/h (c) The amount of product, kg/h.
Problem 9.22: An aqueous solution containing 15 % NaOH and 0.5 % NaCl is concentrated at a rate of 100 kg/min in an evaporator. The concentrated solution is then mixed with 2000 kg of aqueous NaOH solution in a mixer. At the end of one hour, a sample is collected from the mixer and analyzed. The analysis shows 40 % NaOH and 0.8571 % NaCl. Calculate the following: (a) The concentration of the aqueous solution in the mixer (b) The composition of the concentrate from the evaporator (c) The mass of water (in kilograms) evaporated in one hour.
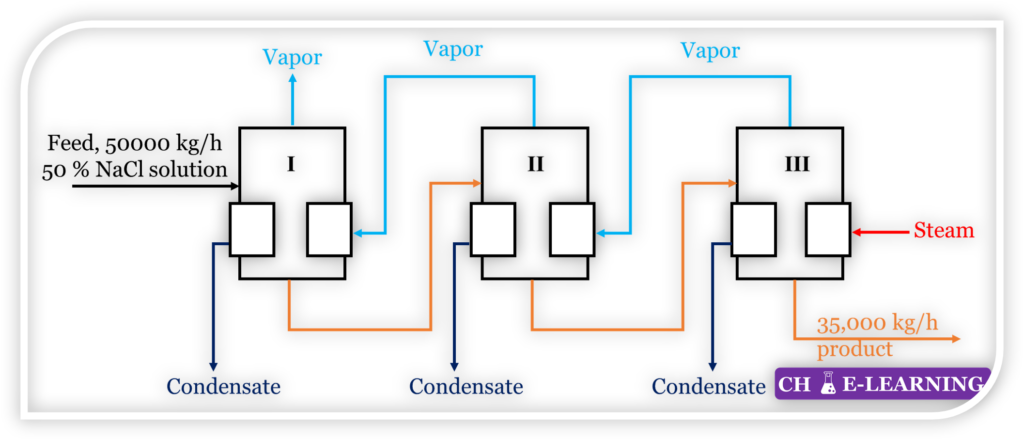
Problem 9.23: A 50 % NaCl solution is to be concentrated in a triple-effect evaporator. An equal amount of water is evaporated in each effect. Determine the composition of the outlet stream from effect II.
Problem 9.24: A crude salt when dissolved in water yields brine whose composition is 15 % by weight of NaCl, 1 % NaBr, and 3 % MgCl2. Some water is evaporated and 40 % of salt (NaCl) crystallizes in pure form. On evaporation, brine loses 70 % water. For 100 kg of original brine, calculate (a) the weight % of MgCl2 and NaBr in the concentrate and (b) The composition of the cake if all the water is evaporated.
Problem 9.25: An evaporator is fed continuously with 50000 kg/h of a solution containing 10 % NaOH, 10 % NaCl, and the rest water by weight. During evaporation, water is removed as vapor and salt NaCl precipitates as crystals and is removed by filtration. The concentrated liquor leaving the evaporator contains 50 % NaOH, 2 % NaCl, and the rest water. Determine (a) the mass of water evaporated per hour (b) the mass of salt precipitated per hour (c) the mass of concentrated liquor produced per hour.
Problem 9.26: 0.05 kg of a slightly soluble salt is mixed with 0.1 kg of water. The undissolved salt is removed by filtration. The filter cake weighed 0.045 kg as obtained and 0.040 kg after drying. What is the solubility of the salt in water expressed in kg salt/100 kg water? What assumptions are inherent in the solution?
Problem 9.27: Fifty kilograms of dry sodium bicarbonate is to be crystallized and removed from 1000 kg of a saturated solution at 333 K. To what temperature the solution be cooled, if the solubility data is as follows?
| Temperature (K) | 333 | 323 | 313 | 303 | 293 | 283 |
| Solubility (kg bicarbonate/100 kg water | 16.4 | 14.5 | 12.7 | 11.1 | 9.6 | 8.2 |
Problem 9.28: 200 kg of 15 % and 100 kg of 5 % solutions of sodium sulfate by weight are mixed in a crystallizer and crystallization takes place. If 50 kg Na2SO4.10H2O crystals are formed, compute the composition of the magma.
Problem 9.29: One hundred kilograms of a mixture of Na2CO3·10H2O and Na2SO4.10H2O is heated to drive away the water of hydration. The anhydrous salt mixture weighed 39.335 kg. What is the mole ratio in which the two salts are present in the mixture?
Problem 9.30: A 10-kg mixture of Ca(NO3)2.4H2O and CuSO4.5H2O is heated to drive off the water of hydration. The residue weighed 6.5591 kg. Determine the percent composition of the mixture of hydrated salts.
Problem 9.31: A saturated solution of calcium chloride in water is to be prepared by dissolving CaCl2.6H2O in 100 kg water at 293 K. If the solubility of calcium chloride in water at 293 K is 75 kg of anhydrous salt per 100 kg of water, what mass of hydrated crystals is required?
Problem 9.32: If 0.1 kg of Na2SO4 is dissolved in 0.2 kg of water and the resulting solution is cooled until 0.1 kg of Na2SO4.10H2O crystallizes out. Determine (a) The composition of the mother liquor (b) The amount of anhydrous crystals obtained per 0.1 kg original solution.
Problem 9.33: What will be the yield of hypo (Na2S2O3·5H2O) if 100 kg of a 50 % solution of Na2S2O3 is cooled to 293 K. The solubility at 293 K is 70 parts Na2S2O3 per 100 parts water.
Problem 9.34: After a crystallization process, a solution of CaCl2 in water contains 62 kg of salt per 100 kg of water. Calculate the weight of the solution necessary to dissolve 250 kg of CaCl2.6H2O at 298 K. The solubility at 298 K is 7.38 kmol CaCl2 in 1000 kg of water.
Problem 9.35: A saturated solution of barium nitrate is to be prepared from 100 kg of the salt at 373 K. (a) Determine the amount of water required (b) If this solution is cooled to 273 K, how much Ba(NO3)2 crystals will be obtained if the precipitated crystals carry 4 kg of water per 100 kg of dry crystals?
The solubility data of Ba(NO3)2 in water: at 273 K, 5.0 kg/100 kg water; at 373 K, 34 kg/100 kg water.
Problem 9.36: A saturated solution of sodium chloride is prepared at 373 K using 100 kg of salt. (a) How much water is required (b) If the solution is cooled to 273 K, how much salt is precipitated out of the solution?
The solubility of sodium chloride is 39.8 kg/100 kg water at 373 K and 35.7 kg/100 kg water at 273 K.
Problem 9.37: 9.37 A batch of 1000 kg of KCl is dissolved in sufficient water to make a saturated solution at 363 K (solubility is 35 wt% KCl in water). The solution is cooled to 293 K, at which its solubility is 25.4 wt%. (a) What is the weight of water required for solution and the weight of crystals of KCl obtained? (b) What is the weight of crystals obtained if 5 % of the original water evaporates on cooling?
Problem 9.38: A salt solution weighing 10000 kg with 30 wt% Na2CO3 is cooled to 293 K. The salt crystallizes as decahydrate. (a) What will be the yield of Na2CO3.10H2O crystals if the solubility is 21.5 kg of anhydrous Na2CO3/100 kg of total water if no water is evaporated? (b) What will be the yield if 3 % of the total weight of the solution is lost by evaporation in cooling?
Problem 9.39: A hot solution of Ba(NO3)2 from an evaporator contains 30.6 kg of Ba(NO3)2 per 100 kg of water and goes to a crystallizer where the solution is cooled and Ba(NO3)2 crystallizes. On cooling 10 % of the original water present evaporates. For a feed solution of 100 kg, calculate the following: (a) The yield of crystals if the solution is cooled to 290 K, if the solubility is 8.6 kg Ba(NO3)2/100 kg total water at 290 K (b) The yield if cooled to 283 K, if the solubility is 7.0 kg Ba(NO3)2/100 kg total water at 283 K.
Problem 9.40: A solution of sodium sulfate in water is saturated at a temperature of 313 K. Calculate the weight of crystals and the percentage yield obtained when cooling 1000 kg of this solution to a temperature of 278 K. At 278 K decahydrate is the stable crystalline form. The solubility at 313 K is 32.6% and at 278 K is 5.75% Na2SO4.
Problem 9.41: A saturated solution containing 1500 kg of potassium chloride at 360 K is cooled in an open tank to 290 K. If the specific gravity of the solution is 1.2, the solubility of KC1 per 100 parts of water is 53.55 at 360 K and 34.5 at 290 K, calculate (a) The capacity of the tank required (b) The weight of crystals obtained neglecting the loss of water by evaporation.
Problem 9.42: Sodium carbonate is recovered as decahydrate from 1000 kg of a 5 % solution of sodium carbonate in water. If it is desired that 95 % of Na2CO3 is to be recovered as decahydrate by cooling to 278 K, determine the following: (a) The mass of water evaporated (b) The mass of crystals obtained
The solubility of Na2CO3 in water at 278 K is 9.0 % (weight).
Problem 9.43: One thousand kilograms of a 30% solution of Na2CO3 in water is cooled slowly to 293 K. During cooling, a portion of water is evaporated and removed. 700 kg of Na2CO3.10H2O crystals are formed in the process. What percent of the total water in the feed is evaporated? The solubility of anhydrous Na2CO3 at 293 K is 21.5 kg/100 kg of water.
Problem 9.44: An aqueous solution of Na2CO3 contains 12 % carbonate and small amounts of soluble impurities. 80 % of the carbonate is recovered as Na2CO3.10H2O by evaporation of water and subsequent cooling to 278 K. The solubility of Na2CO3 at 278 K is 9.0 % (weight). On the basis of the 100 kg solution treated, determine the following: (c) The amount of water evaporated (d) The quantity of crystals formed.
Problem 9.45: In the recovery of glycerine from spent soap lye, 10000 kg/h of lye containing 10.5 % glycerine and 11.5 % salt (NaCl) is concentrated by evaporation. The concentrated solution leaving the evaporator contained 80 % glycerine and 5 % salt. Entrainment loss is estimated to be 2 % of the glycerine in the charge. Calculate the quantity of (a) The water evaporated per hour (b) The salt crystallized per hour.
Problem 9.46: A solution containing 25 % MgSO4 and 75 % water is cooled so that MgSO4·7H2O crystals are obtained on crystallization. During this process, 6.5 % of the total water present in the feed is lost due to evaporation. The solubility of anhydrous MgSO4 in water at this temperature is such that 35 % of the anhydrous salt is present in the saturated solution. For treating 1000 kg of solution, calculate the following: (a) The weight of MgSO4.7H2O crystals obtained (b) The solubility of anhydrous salt in water (kg salt/100kg water).
Problem 9.47: The solubility of NaNO3 in water is 1.76 kg/kg water at 373 K and 0.88 kg/kg water at 293 K. A 25 % (weight) solution of NaNO3 in water is concentrated to saturation by evaporation at 373 K. It is then cooled to 293 K. The crystals formed are separated. It is found that each kilogram of crystals so removed carries with it 0.1 kg of solution. When the crystals are dried, NaNO3 in the adhering solution gets deposited on the crystals. For 1000 kg of the original dilute solution, calculate the following: (a) The amount of water evaporated for attaining saturation at 373 K (b) The weight of dry crystals obtained.
Problem 9.48: A dilute salt solution containing 6 % salt is fed to an evaporator at a rate of 10000 kg/ h for a trial run. Crystals formed along with the saturated solution adhering with them are sent to a centrifuge for the recovery of the solution. 60 % of the adhering solution is recovered. It is found that the saturated solution that is withdrawn from the evaporator is 850 kg and that is recovered by centrifuging is 200 kg. The crystals are dried to drive off the remaining water. The dry crystals obtained weighed 360 kg. Determine the following: (a) The solubility of the salt in kg salt/100 kg water (b) The mass of salt crystallized in the evaporator (c) The amount of water evaporated in the evaporator (d) The amount of water removed in the drier.
Problem 9.49: An aqueous solution containing 60 % Na2S2O3 and 1 % soluble impurities is diluted with water and fed to a crystallizer where it is cooled to 283 K in order to crystallize Na2S2O3.5H2O. The crystals carry 0.06 kg of solution (excluding impurities) per kg of crystals. The free water present in the adhering solution is removed on drying the crystals. The final dried product contains not more than 0.1% impurity. The solubility of the pentahydrate is 1.4 kg of Na2S2O3.5H2O/kg free water. On the basis of 100 kg of 60 % solution, calculate the following: (a) The mass of water added before cooling (b) The percentage recovery of the Na2S2O3 in the dried hydrated crystals.
Problem 9.50: Oilseeds containing 40 % oil and the rest inert insoluble are extracted with hexane to recover oil. Oil is dissolved in the solvent and is removed as a clear solution. The underflow sludge analyzed 10.53 % oil and 26.32 % hexane. Identify the key component. Determine the percent recovery of oil.
Problem 9.51: Tannin is extracted from certain wood bark which contains 6 % moisture, 11.0 % tannin, 8.0 % soluble non-tannin materials, and the rest insoluble lignin. After tannin is extracted, the solid residue analyses 1 % tannin and 0.25 % soluble non-tannin on a dry basis. What is the percent extraction of tannin?
Problem 9.52: Black ash containing 45 % Na2CO3, 5 % water-soluble materials, and 50 % inert insoluble is extracted with water to recover soda ash. The solid residue leaving the extraction unit contains 5 % Na2CO3, 0.5 % water soluble materials, 85 % insoluble, and the rest water. Calculate per 1000 kg of black ash treated (a) The mass of residue produced (b) The mass of sodium carbonate extracted.
Problem 9.53: Oil is extracted from seeds by leaching with organic solvents. Soybean seeds containing 20 % oil, 65 % inert solids, and 15 % water are leached with hexane and after extraction, the solid residue is removed from the solution of oil in hexane. The residue analyzed was 1.0 % oil, 88 % inert cake, and 11 % water. What percent of the oil in the seeds is recovered?
Problem 9.54: A counter-current extractor is employed to extract oil from a solid meal using ethyl ether as the solvent. The fresh meal is charged to the unit at a rate of 1000 kg/h and contains 25.0 % (weight) oil. Pure solvent enters the bottom of the extractor. The overflow from the unit contains 60 % (weight) oil. The underflow contains 0.25 kg of solution per kg of oil-free solids and the concentration of oil in the underflow is 12 %. Calculate the following: (a) The solvent requirement (b) The percentage of oil recovery.
Problem 9.55: 100 kg of ore containing 70 % solute and 30 % inert solid is to be extracted by washing it twice with 50 kg of batches of fresh water by a simple multiple contact method. The underflow retains 0.6 kg of solution per kg of inert solid. Calculate the following: (a) Concentration of solute in the final underflow (b) Concentration of the solute in the combined extract (c) Percentage recovery of the solute after two washings.
Problem 9.56: Soap as produced contains 50 % moisture on a wet basis. Before it can be pressed into cakes for sale, the moisture should be reduced to 20 %. How many 100 g cakes can be pressed from 1000 kg of wet soap?
Problem 9.57: A wet paper pulp containing 70 % water is dried in order to remove 60 % of the water present. Determine the following: (a) The mass of water removed per 100 kg of wet pulp (b) The composition of the dried pulp.
Problem 9.58: A wet granular material containing 80 % water is dried in a rotary counter-current drier. The charge is admitted at one end and hot dry air is passed from the other end. In a typical operation, it is found that 100 kg of water is removed from the material giving a dry product containing 40 % water. What is the weight of the wet material charged to the drier?
Problem 9.59: Wood containing 40 % moisture is dried to 20 % moisture, and both moistures are expressed on a wet basis. Determine the quantity of water evaporated per kilogram of dry wood.
Problem 9.60: A batch of leather leaving a drier weighs 1000 kg and contains 5 % moisture. During drying the leather loses 50 % of its original weight. Determine the following: (a) The moisture content of the leather entering the drier on a dry basis (b) The amount of moisture removed per kg of bone-dry leather (c) Water removed as a percent of the original water present.
Problem 9.61: A drier is fed with a wet solid to reduce the moisture content from 90 % to 20 %. The product leaving the drier is admitted to an oven which further brings down the moisture to 2 %. If the drier can handle 1000 kg of wet solids per day, calculate the following: (a) The weight of products leaving the drier and the oven per day (b) The percentage of the original water that is removed in the drier and the oven.
Problem 9.62: Air supplied to an adiabatic drier is at 101.3 kPa and 369.15 K with a dew point of 301.15 K. 2.0 kg of water is evaporated per 100 cubic meters of wet air entering the drier. Calculate the following: (a) The exit air wet-bulb temperature and dry-bulb temperature (b) The exit air percent humidity (c) The volume of exit air per 100 cubic meters of inlet air.
Problem 9.63: A laboratory drier produces 50 kg of dry solid containing 6 % (weight) water from a wet-feed material containing 20.5 % water. The drier operates adiabatically, with the wet solid entering the drier at the wet-bulb temperature of the drier air. A total of 500 m3/ h of hot air at 365.7 K and 101.3 kPa with a dew point of 303.2 K is supplied to the drier. Calculate the following: (a) The temperature of the air leaving the drier (b) The percent humidity of the air leaving the drier.
Problem 9.64: Air at 101.3 kPa enters an adiabatic drier at 372 K with a dew point of 287 K and leaves at 80 % humidity. Wet paper enters the drier with 25 % moisture and leaves with 5 % moisture. Determine the following: (a) The temperature of the air leaving the drier (b) Water evaporated in kilograms per 100 cubic meters of air entering (c) The mass of the finished product (in kilograms) per 100 cubic meters of air entering.
Problem 9.65: Wet lumber (5 % moisture) is dried to 1 % moisture in a hot-air drier. Air fed to the drier contains 0.5 % water. The moist air leaving the drier contains 2 % (weight) water. How much air is required to dry 2000 kg/h of lumber?
Problem 9.66: 1000 kg/h of precipitated chalk slurry containing 10 % CaCO3 is to be filtered and dried continuously. The filter cake is expected to carry 0.8 kg of water per kg of chalk. Hot air would enter the adiabatic drier at 350 K 1 bar and 5 % humidity. At the exit, the air would be saturated. The dried chalk would contain only 5 % water on a wet basis. Calculate on an hourly basis the following: (a) The weight of the filtrate (b) The weight of the cake after dying (c) The weight of air at the drier inlet.
Problem 9.67: CaCO3 slurry is to be dried. The drier is designed to remove 100 kg of moisture per hour. Air at 293 K and 40 % relative humidity enters the drier and leaves at 338 K and 65 % relative humidity. (a) What is the weight in kilograms of bone-dry air required per hour? The atmospheric pressure is 103 kPa. (b) If the humidity of the air entering the drier can be varied, what will be the minimum amount of dry air required? The constants for the Antoine equation for the vapor pressure of water in kPa may be taken as A = 16.26205, B = 3799.887, and C = -46.854.
Problem 9.68: A solid material wet with toluene is dried in an adiabatic drier to recover toluene and to produce dry solids. The drier is fed with 100 m3/h of an air-toluene mixture at 101.3 kPa and 333 K with a wet-bulb temperature of 305 K. The gases leave the drier at 310 K DBT with a relative saturation of 95 %. The vapor pressure of toluene is given by the Antoine equation
\ln P^S=13.9987-\frac{3096.52}{T-53.67}where pressure is in kPa and temperature is in K. The latent heat of vaporization is 404 kJ/kg and the psychrometric ratio for the toluene-air system is 1.88 kJ/kg K. Determine the following: (a) The toluene evaporated in the drier, (kg/h) (b) The volume of gases leaving the drier, (m3/h).
Problem 9.69: Two liquids A and B are only partially miscible. At a certain temperature, 41 kg of A and 59 kg of B are mixed well and the mixture is allowed to settle. The mixture separates into two immiscible phases, one rich in A and the other rich in B. The A-rich phase analyses 90 % A and the B-rich phase analyses 80 % B. What are the weights of the A-rich and B-rich phases?
Problem 9.70: A mixture of phenol and water under certain conditions of temperature and composition forms two separate layers, one rich in phenol and the other rich in water. At 300 K, the composition of the upper and lower layers are 70 % and 9 % by weight of phenol respectively. If 40 g of phenol and 60 g of water are mixed and the layers are allowed to separate at 300 K, what will be the weights of the two layers?
Problem 9.71: Acetic acid is extracted from an aqueous solution containing 42.86 % (weight) acetic acid using benzene as the solvent. The mutual solubility of benzene and water may be neglected. When equal weights of benzene and the aqueous solution are mixed and the phases separated, the aqueous phase analyzed 16.25 % acetic acid. On the basis of 100 kg of the aqueous solution being treated, calculate the following: (a) The masses of the aqueous phase and benzene phase (b) The percent recovery of acetic acid into benzene.
Problem 9.72: Toxic impurities A and B present in an oil are to be removed before it is subjected to hydrogenation. 1000 kg of oil is being charged. A portion of this oil containing 3 % A and 2 % B is sent through a purifier where it is treated with a mixture of two solvents S1 and S2. 100 kg of S1 can dissolve a maximum of 15.0 kg of A and 2 kg of B whereas 100 kg of S2 can dissolve a maximum of 3 kg of A and 12 kg of B. The solvent mixture containing dissolved impurities is then separated from the oil. The oil free of impurities is mixed with a portion of the original feed that has bypassed the purifier and sent to the hydrogenation unit. The feed to the hydrogenator should contain A and B not more than 0.1 % each. Calculate the following: (a) The amounts of solvents S1 and S2 required (b) The amount of oil bypassed the purifier.
Problem 9.73: A mixture containing 30 % acetone and 70 % chloroform is extracted with a mixed solvent containing acetic acid and water. The two immiscible phases-the raffinate and extract phases that result after extraction had the following analysis:
Extract: acetone 7.5 %, chloroform 6.06 %, acetic acid 31.88 % and water 54.56 %
Raffinate: acetone 20.0 %, chloroform 67.0 % acetic acid 10.0 %, and water 3.0 %
For the basis of 100 kg of the mixture extracted, determine the following: (a) The composition of the mixed solvent on a weight basis (b) The quantities of raffinate and extract phases (c) The amount of mixed solvent used.
Problem 9.74: Ammonia is recovered from a gas mixture containing 25 % (volume) CO2 and 75 % (volume) NH3 by scrubbing with water. Assuming that CO2 is insoluble in water, determine the percent of ammonia in the entering gas that is absorbed if the gas leaving the scrubber analyses 35 % NH3.
Problem 9.75: Air at 540 K and 101.3 kPa is dried from a partial pressure of 7000 Pa of water vapor to a partial pressure of 1500 Pa of water vapor at constant total pressure. How much water in kilograms is removed per cubic meter of wet air entering?
Problem 9.76: A stream of gas at 302 K and 100 kPa, 50 % saturated with water vapor is passed through a drying tower where 90 % of the water vapor is removed. For 100 cubic meters of gas admitted through the tower, how many kilograms of water are removed? The vapor pressure of water at 302 K is 4.0 kPa.
Problem 9.77: One hundred kilograms per hour of 98 % (weight) sulphuric acid is used for the absorption of SO3 in the contact sulphuric acid plant. If 20 % oleum is the product leaving the absorption tower, what mass of SO3 is absorbed per hour?
Problem 9.78: A gas mixture at 300 K and 1 bar analyzing by volume 20 % N2 and 80 % CH4 is subjected to liquefaction at a rate of 1500 kg/h. It is found that only 30 % (weight) of the entering gas is liquefied and the concentration of N2 in the liquid is 60 % by weight. The unliquefied gas leaves the unit at 273 K and 1 bar. Determine (a) the volume of unliquefied gas, m3/h (b) the composition of the gas leaving expressed as volume %.
Problem 9.79: Hydrogen sulfide is absorbed from a gaseous mixture containing 26 % H2S and 74 % inerts by a solution in a tower. The tower operates at 4 bar, and 330 K. The gases leave the tower with an H2S content of 8 %. Assuming that H2S is alone removed and nothing is added as the gases pass through the tower and if the feed to the tower is 3000 m3/h, calculate (a) The amount of H2S recovered from the gas and (b) The percentage recovery of H2S.
Problem 9.80: A gas mixture consisting of 60 % N2 and 40 % SO3 is admitted to an absorption column at a rate of 100 kmol/h. It is contacted with a stream of 50 % H2SO4 flowing counter-current to the gas stream at a rate of 6000 kg/h. The gases leave at 101.3 kPa. The water lost with the exit gases exerts a partial pressure of 26.66 kPa. If the concentrated acid leaving the bottom of the column contains 74.73 % H2SO4 what percent of the entering SO3 will be absorbed and converted to acid?
Problem 9.81: In the preparation of cooking liquor for a sulfite pulp mill, an absorption column is used to absorb SO2 in a weak liquor. The weak liquor enters the top of the column at a rate of 1.5 m3/min with an SO2 concentration of 0.5 % (weight) and leaves with an SO2 concentration of 1.0 % (weight). The gas stream entering the bottom of the column and passing in the counter-current direction to the liquid stream contains 15 % SO2. When the gas leaves the top of the column, 80 % SO2 is absorbed. The pressure in the column is 1.5 bar and the temperature is 310 K. Assuming that the liquor has a specific gravity of 1.0, determine the following: (a) The amount of SO2 absorbed per minute (b) The molar flow rate of gas entering the absorber (c) The percentage of SO2 in the gas leaving the column (d) The volumetric flow rate of gas leaving the absorber.
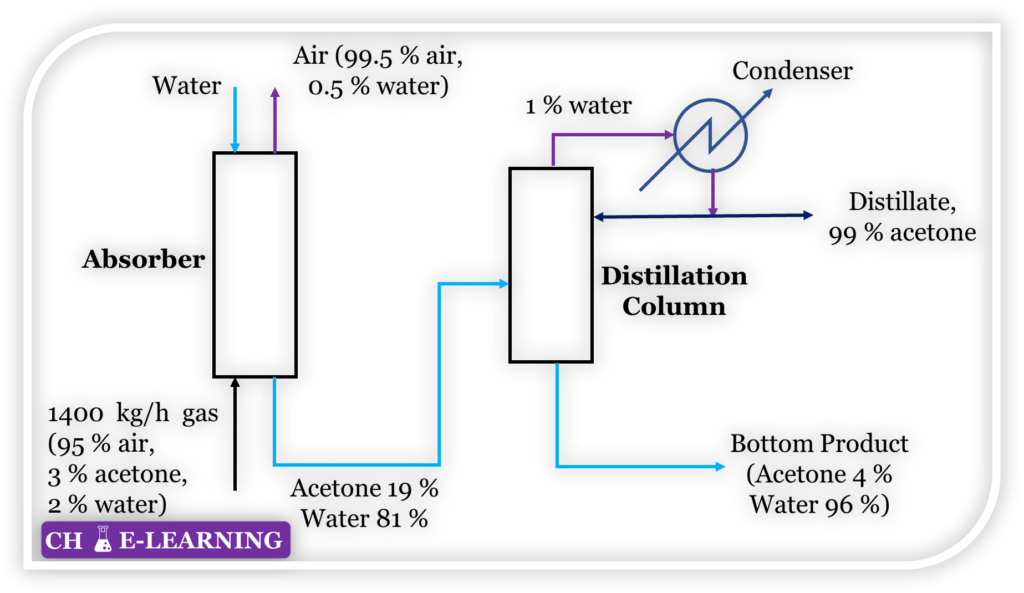
Problem 9.82: Figure represents the flow sheet for the recovery of acetone from air. All compositions are on a weight basis. Make a material balance and determine the quantities of the following streams: (a) Water added in the absorber (b) Acetone-free air leaving the absorber (c) Aqueous solution of acetone leaving the absorber (d) Distillate product (e) Bottom product
Problem 9.83: A mixture of benzene and toluene containing 10 % by mole benzene is continuously distilled at a rate of 1000 kmol/h in a distillation column. 95 % of the benzene in the feed is recovered as a distillate product which contains 98 % benzene and 2 % toluene. Calculate the following: (a) The moles of the bottom product (b) The composition of the bottom product.
Problem 9.84: An aqueous solution of ethanol containing 10 % by weight ethanol is continuously distilled at a rate of 1000 kg/h in a distillation column. Ten percent of the feed is recovered as distillate product which contains 60 % ethanol and 40 % water. Calculate the following: (a) The weight of alcohol lost in the bottom product (b) The composition of the bottom product.
Problem 9.85: A distillation column is charged with an aqueous solution of ethanol containing 35 % ethanol by weight. The concentrated alcohol is withdrawn as the distillate containing 85 % alcohol. The bottom product (residue) contains 5 % ethanol. Determine the following: (a) The mass of distillate per 100 kg of feed (b) The ratio of the mass of the distillate to the mass of the residue.
Problem 9.86: A continuous distillation column is used to regenerate solvent for use in a solvent extraction unit. The column treats 100 kmol/h of feed containing 15 % (mol) ethyl alcohol and the rest water. The overhead product is 89.43 % alcohol and the bottom product is 0.5 % alcohol. The overhead is sent to the extraction unit and the bottom is wasted. What is the daily requirement of make-up alcohol in the solvent extraction unit?
Problem 9.87: A benzene-toluene solution containing 40 % (weight) benzene is fed into the distillation column. A distillate product that is rich in benzene leaves the top of the column and a residue that is rich in toluene leaves the bottom of the column. The distillate contains 97 % (weight) benzene and the bottom product contains 95 % (weight) toluene. Calculate the following: (a) The composition of the feed, distillate, and the bottom product in mole percent (b) The moles of distillate and the bottom product obtained by separating 100 moles/hour of the feed.
Problem 9.88: One thousand kilograms per hour of a hydrocarbon mixture consisting of 40 % benzene, 40 % toluene, and 20 % xylene is admitted to the first column of a series of two distillation columns. The top product from this column contains 99 % benzene and 1 % toluene. The bottom product enters the second column in the series where it is subjected to further purification. The distillate leaving the second column is 95 % toluene and 5 % benzene and the bottom product is 90 % xylene and 10 % toluene. Calculate (a) the quantity and (b) the composition of the bottom product from the first column. All concentrations are on a weight basis.
Problem 9.89: One hundred moles of a hydrocarbon mixture consisting of 20 % ethane, 40 % propane, and 40 % butane is admitted to the first column of a series of two distillation columns. The top product from this column contains 95 % ethane, 4 % propane, and 1 % butane. The bottom product enters the second column in the series where it is subjected to further purification. The distillate leaving the second column is 99 % propane and 1 % butane and the bottom product is 8.4 % propane and 91.6 % butane. Calculate (a) the quantity and composition of the bottom product from the first column and (b) the quantity of the distillate from the second column.
Problem 9.90: The feed to a distillation column is separated into net overhead products containing nothing with a boiling point higher than that of isobutane and bottoms containing nothing with a boiling point below that of propane. The composition of the feed is
| Component | Ethylene | Ethane | Propylene | Propane | Isobutane | n-Butane | n-Pentane |
| mol% | 2.0 | 3.0 | 5.0 | 15.0 | 25.0 | 35.0 | 15.0 |
The composition of isobutane in the overhead is 5.0 mol%, and the concentration of propane in the bottom is 0.8 mol%. Calculate the composition of the overhead and bottom streams per 100 moles of feed.
Problem 9.91: Refer to Figure. Feed enters the first column of the two-stage unit with a flow rate of 100 kg/s. The top product from this column is withdrawn at a rate of 40 kg/s.
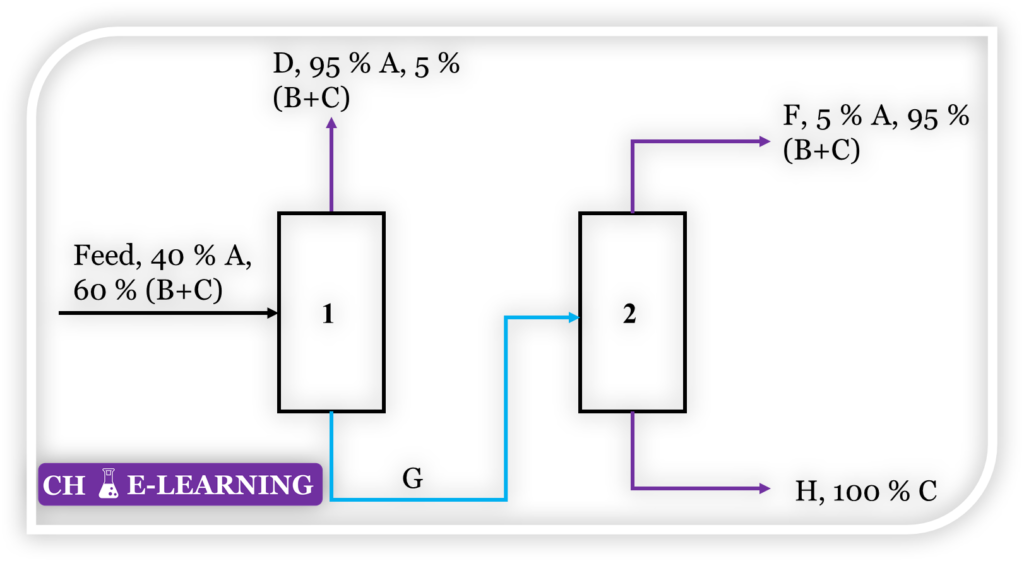
Streams D and F contain B and C in the ratio 10 1 by weight. All percentages are on a weight basis. Determine the following: (a) Flow rates of streams F, G, and H (b) Percent of B and C in the feed, D and F.
Problem 9.92: Oxygen is produced by liquefying air and distilling it at low temperatures. Liquefied air (21 % O2, 79 % N2) is sent to a distillation column where it is separated as stream 1 (60 % N2, 40 % O2) leaving as the bottom and stream 2 (96 % N2, 4 % O2) leaving at the top. Both streams are then fed to another column. Nitrogen-rich product (98 % N2, 2 % O2) is withdrawn as distillate and oxygen-rich product (99.5 % O2, 0.5 % N2) is withdrawn as the bottom product. Refer to Figure.

Calculate the following: (a) The percent recovery of oxygen in the oxygen-rich product (b) The flow rate of stream 1 in mol/s for the production of the oxygen-rich product at a rate of 3.2 kg/s.
