What Remains Conserved Out of Mass, Mole & Gram-atom During Any Process?
In this post, you will learn what remains conserved when (1) there is no chemical reaction, (2) there is a chemical reaction, and (3) there is a nuclear reaction.
Case 1: When there is no Chemical Reaction
Consider an example of the distillation column.
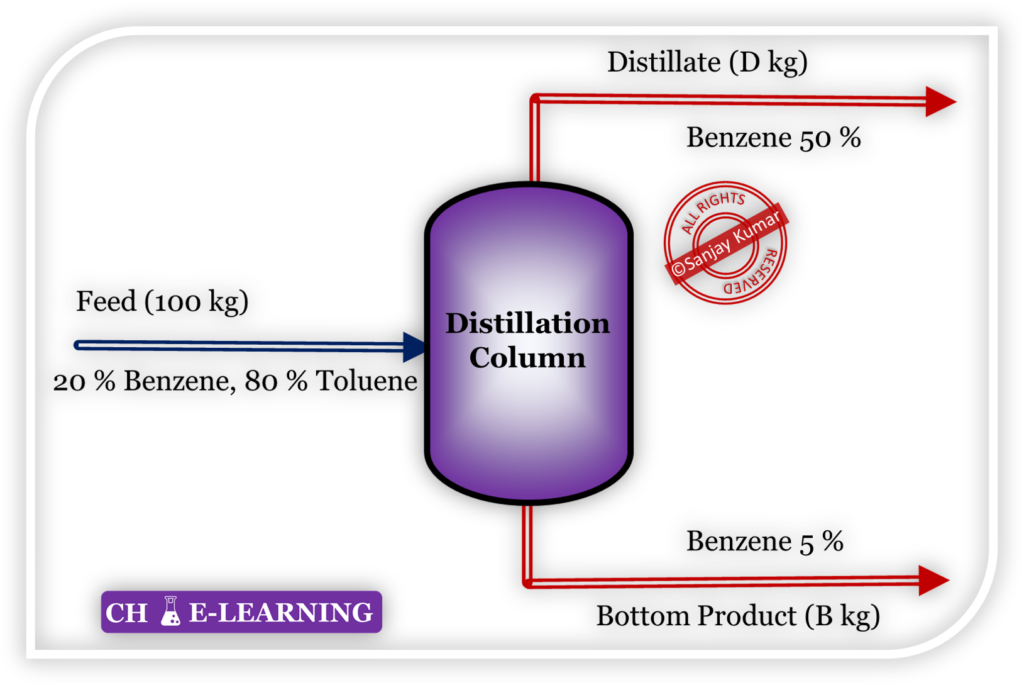
- First, apply the total mass balance
- Apply mass balance for benzene
- From equations (1) & (2),
Wt. of benzene (distillate side) \mathrm{=33.33\times\frac{50}{100}=16.67\;kg}
Wt. of benzene (bottom product side) \mathrm{=66.67\times\frac{05}{100}=3.33\;kg}
wt. of benzene (feed side) = wt. of benzene (distillate side) + wt. of benzene (bottom side)
\mathrm{20\;kg=16.67+3.33=20\;kg \Rightarrow Mass\;is\;conserved}
Molecular weight of benzene = 78 g/mol; Molecular weight of toluene = 92 g/mol
Moles of benzene (feed side) \mathrm{=\frac{20\;kg}{78\;g/mol}=256.41\;mol}
Moles of benzene (distillate side) \mathrm{=\frac{16.67\;kg}{78\;g/mol}=213.72\;mol}
Moles of benzene (bottom product side) \mathrm{=\frac{3.33\;kg}{78\;g/mol}=42.69\;mol}
Moles of benzene (feed side) = Moles of benzene (distillate side) + Moles of benzene (bottom side)
\mathrm{256.41=213.72+42.69=256.41 \Rightarrow Moles\;is\;conserved}
\mathrm{256.41=213.72+42.69=256.41}Conclusion
- When there is no chemical reaction, mass, moles & gram-atom remain conserved.
Critical Thinking
Here, benzene (C6H6) is not losing its identity. In the distillate and bottom side, benzene remains C6H6.
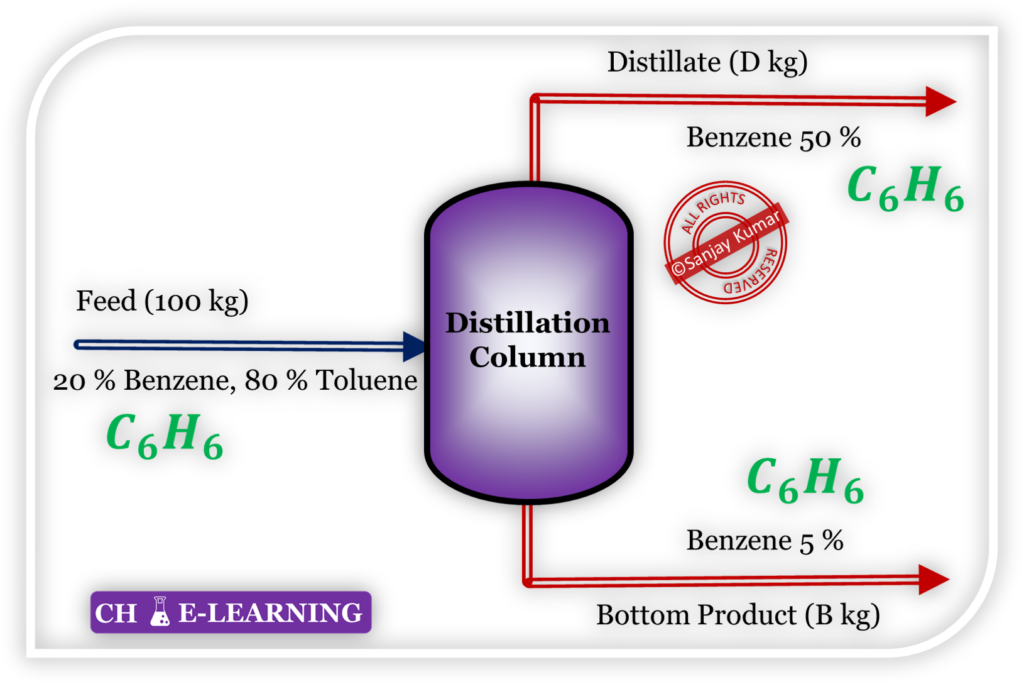
- Whenever a compound does not lose its identity then both mass, as well as mole balance, can be applied.
Case 2: When there is a Chemical Reaction
Consider an example of a simple reaction

- During chemical reactions, molecules lose their identity. However, the identity of elements remains intact.
| Reactants side | Product side |
| 1 gram-atom of C | 1 gram-atom of C |
| 4 gram-atom of H | 4 gram-atom of H |
| 4 gram-atom of O | 4 gram-atom of O |
| Total 9 gram-atom | Total 9 gram-atom |
\mathrm{Gram‐atom\;(reactant\;side)\;=\;Gram‐atom\;(product\;side)}
In a reaction, gram-atom remains conserved.
Total no. of moles (reactant side) =1+2=3 ; Total no. of moles (product side) =1+2=3
\mathrm{Total\;moles\;(reactant\;side)\;=\;Total\;moles\;(product\;side)}
- In this reaction, moles are conserved. Is it always true for all reactions? We’ll find out later.

Total mass of reactants \mathrm{=16\;g+64\;g=80\;g} ; Total mass of products \mathrm{=44\;g+36\;g=80\;g}
\mathrm{Total\;mass\;(reactant\;side)\;=\;Total\;mass\;(product\;side)}
- In this reaction, gram-atom, mass & moles are conserved. Further, we will verify it by taking another reaction.
Consider another reaction

| Reactants side | Product side |
| 4 gram-atom of H | 4 gram-atom of H |
| 2 gram-atom of O | 2 gram-atom of O |
| Total 6 gram-atom | Total 6 gram-atom |
\mathrm{Gram‐atom\;(reactant\;side)\;=\;Gram‐atom\;(product\;side)}
Total mass of reactants \mathrm{=4\;g+32\;g=36\;g} ; Total mass of products \mathrm{=2\times18=36\;g}
\mathrm{mass\;of\;reactants\;=\;mass\;of\;products}
Total no. of moles (reactant side) \mathrm{=2+1=3} ; Total no. of moles (product side) =2
\mathrm{moles\;of\;reactants\neq\;moles\;of\;products}
Conclusions
- During a chemical reaction, mass & gram-atom are always conserved. However, no. of moles may or may not be conserved.
Critical Thinking
- Here, molecules are losing their identity during a chemical reaction. However, elements (such as C, H & O) are not losing their identity. So, mass & gram-atom remain to conserve.
Case 3: When there is a Nuclear Reaction (fusion & fission)
Consider a nuclear reaction
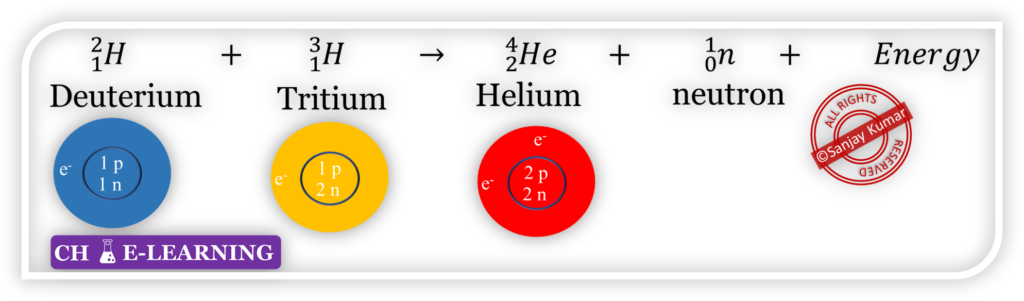
- Here, the identity of elements is no longer intact.
\mathrm{Total\;moles\;(reactant\;side)\;\neq\;Total\;moles\;(product\;side)}
- Weight of Deuterium (2 g/mol) + Weight of Tritium (3 g/mol) ≠ Weight of Helium (4 g/mol)
\mathrm{Total\;mass\;(reactant\;side)\;\neq\;Total\;mass\;(product\;side)}
\mathrm{Gram‐atom\;(reactant\;side)\;\neq\;Gram‐atom\;(product\;side)}
Conclusions
- During a nuclear reaction, total mass & energy before and after nuclear reaction remains conserved.
Critical Thinking
- When an element loses its identity then some mass can convert into energy or vice versa. So, total mass and energy remain conserved.
- This loss in mass can be related to energy using Einstein’s equation
\mathrm{E=mc^2}
- Where m is mass loss or defect mass.

Its like you read my mind You appear to know a lot about this like you wrote the book in it or something I think that you could do with some pics to drive the message home a little bit but instead of that this is fantastic blog An excellent read I will certainly be back
Somebody essentially help to make significantly articles Id state This is the first time I frequented your web page and up to now I surprised with the research you made to make this actual post incredible Fantastic job