Introduction:
Chemical Engineer converts raw materials into useful products economically at a large scale through chemical and physical operations. The raw materials are treated through physical steps to make them suitable for the chemical reaction. After the chemical reaction, products are separated again through physical steps. All these processes are carried out in Chemical Process Industries.
Process:
Series of operations that causes physical and chemical changes in a substance.
- Space, time, expertise, and resources are needed for such processes.
- Depending on the mode of operation, processes can be classified as Batch, Semi-batch, and continuous processes.

- Physical changes come under Unit Operation. Examples are mixing, separation, heating/cooling, absorption, drying, distillation, filtration, screening, crystallization, material handling, size reduction, extraction, etc.

- Chemical changes come under the Unit Process. Examples are the formation of curd from milk, chemical reaction, oxidation, reduction, polymerization, etc.
Batch process:
A batch process does not deliver its product continuously. It receives raw materials at the beginning of the process and produces products after the completion of the process. Examples are pharmaceutical production, food processing, alcohol fermentation, soap manufacturing, etc.
- The production rate is less as compared to a continuous process. Therefore, small-scale processes can be carried out under batch operation.
- The batch process can be controlled easily. It requires less manpower and energy.
- The batch process operates under unsteady state conditions. Consider the process of making pickles.

Unsteady state condition:
Process variables changes with time. In the above example, the taste and color of the pickle change with time.
Semi-batch process:
A process that is neither batch nor continuous. Examples are fermentation with purge and drug production.
- In semi-batch, either raw materials are periodically added or products are periodically removed.
- The semi-batch process operates under unsteady state conditions.
Advantages of using Semi-batch:
- In the case of exothermic reactions, the temperature of a reactor can be controlled easily by adding reactants slowly.
- In the case of a reversible reaction, the reaction rate can be favored in the forward direction by removing products via the purge stream.
Continuous process:
A continuous process delivers its product continuously. It continuously receives raw materials and produces products. Examples of distillation processes are the production of sulfuric acid, nitric acid, etc.
- The production rate is more, it can run 24/7. Therefore, large-scale processes can be carried out under continuous operation.
- The continuous process requires a sophisticated control system. It requires more manpower and energy.
- The continuous process operates under steady-state conditions.
Steady-state condition:
Process variables do not change with time.
Chemist vs. Chemical Engineer:
| Chemist | Chemical Engineer |
|---|---|
| Chemical engineers utilize these materials and chemical processes and upscale them. | Chemical engineer utilizes these materials and chemical processes and upscales them. |
| These laboratory-scale techniques are unsuitable for large-scale productions. | For commercial production, these techniques are transformed along with various physical and chemical operations. |


- Therefore, a large number of physical and chemical steps are required to convert inexpensive raw materials to the desired products.
Manufacturing Processes:
All processes can be classified into a series of unit operations and unit processes.

- In process calculations, we address the mass and energy balances involved in unit operation and processes.
- The behavior of gases and gas mixtures, pure liquids and solutions, etc.
- Use of psychrometric charts, steam tables, PVT diagrams, etc.
Definition of Chemical Engineering:
Chemical engineering is a branch of engineering that uses principles of chemistry, physics, mathematics, and economics to produce chemicals, useful materials, and energy at a mass scale.
- It can take the smallest of discoveries in laboratories from all fields of science and technology and replicate them on a mass scale, consistently and economically.
Historical background of Chemical Engineering:
The Chemical engineering profession began in 1888. At that time, a chemical engineer was either a mechanical engineer who had some knowledge of chemical process equipment, a chemical plant foreman, or an applied chemist with knowledge of large-scale industrial chemical reactions
At the International level:
- In 1880, George Edward Davis tried to unite these varied professionals through a “Society of Chemical Engineers” that proved unsuccessful.
- Chemistry Professor Lewis Norton of MIT introduced “Course X“, thereby uniting chemical engineers through a formal degree.
- The American Institute of Chemical Engineers (AIChE) was founded in 1908.
- In 1915, the concept of Unit operations was first presented by Arthur D. Little.
- In 1923, W. H. Walker, W K Lewis, and W H McAdams put forward the idea of treating various processes of the chemical industry as a series of unit operations in their famous book “Principles of Chemical Engineering”.
At the National level:
- In 1921, the late Dr. H. L. Roy (founding father of chemical engineering) was the first person who introduced chemical engineering to the curriculum of the Bengal National College, the nucleus of the present Jadavpur University.
- Then UDCT Bombay offered BSc course in Textile chemistry and chemical engineering.
- Andhra University college of engineering was established in 1933, offering graduate and postgraduate courses in sugar technology and chemical technology.
- The Indian Institute of Science (IISc) founded in 1909 started offering a course in chemical engineering from the department of general chemistry. In 1951, the chemical engineering section got a separate status.
- Harcourt Butler Technological Institute (HBTI), Kanpur set up in 1921offered training in oil and paints, leather, and sugar technology.
- The Indian Institute of chemical engineers came into being in 1947.
Evolution of Chemical Industries:
Before the 18th Century, Industrial chemicals were mainly produced through batch processing. After the industrial revolution, industrial production shifted from batch to continuous processing.
Production of Sulfuric Acid:
- Sulfuric acid was the first chemical produced in large amounts through the industrial process.
- In the 17th century, the German chemist Johann Glauber produced sulfuric acid by heating sulfur (s) with saltpeter (s) (Potassium nitrate KNO3) in a glass container, in the presence of steam. Acid was collected from condensed vapors. Thermal decomposition of KNO3 followed by oxidation produces SO3, which combines with water to produce acid.
\mathrm{ KNO_3\;\xleftrightarrow{thermal\;decomposition}\;KNO_2\;+\;O_2\;\xrightarrow{oxidation\;of\;S}\;SO_3 }
\mathrm{SO_3\;\;+\;H_2O\;\xleftrightarrow{acid\;formation}\;H_2SO_4}
- In 1736, London pharmacist Joshua Ward produced Sulphur acid on large scale using the same process.
- John Roebuck and Samuel Garbett were the first to establish a large-scale factory in Scotland in 1749. They were able to produce less expensive and stronger sulfuric acid using lead-lined chambers (lead-chamber process).
Process Description (Lead Chamber):
Glover Tower:
Hot sulfur dioxide enters the bottom of the reactor, where it is washed with nitric oxide and nitrogen dioxide gases, and some SO2 is oxidized to SO3.

Lead Chamber:
A mixture of gases is transferred to a series of chambers where it reacts with water. Acid condenses on the wall and collects on the floor of the chamber.
Gay-Lussac Tower:
Gases pass through the tower where they are washed with cooled concentrated acid. Nitrogen oxide and unreacted sulfur dioxide dissolve in the acid to form nitrous vitriol used in the Glover tower.
- Now a more economical process i.e., the Contact process is used, which produces pure and concentrated sulphuric acid.
Process Description (Contact Process):
Furnace:
Sulfur is burned in an excess of air to produce sulfur dioxide.
Converter:
The oxidation of SO2 to SO3 takes place at a high temperature in the presence of a catalyst.
\mathrm{SO_2\;(g)\;+\;O_2\;(g)\;\leftrightarrow SO_3\;(g)\;\;\;\;\;\triangle H=-197KJ/mol(exothermic)}
NOTE: The use of catalysts only speed-up the reaction rate it has nothing to do with the position of thermodynamic equilibrium.
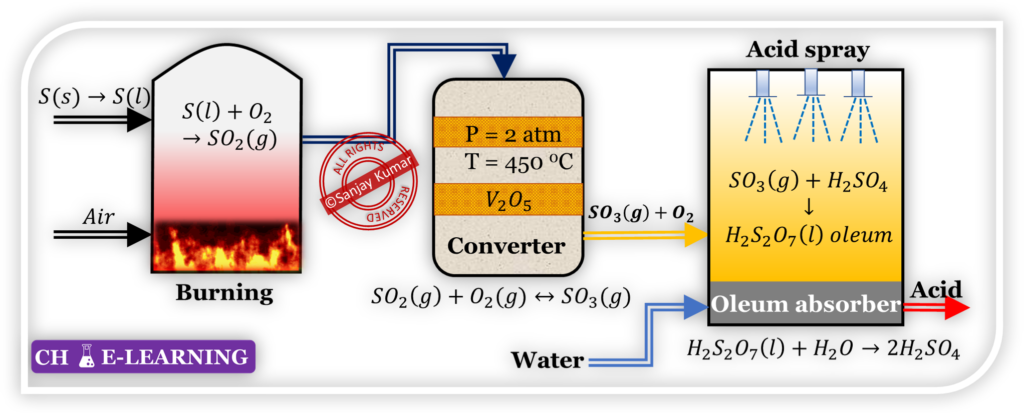
Absorber:
Sulfur trioxide and water react uncontrollably. Therefore, SO3 is first dissolved in acid to make oleum. Then, the oleum reacts with water to produce acid.
- Today the largest use of sulfuric acid is in the manufacture of fertilizers. It is also necessary for petroleum purification, steel production, electroplating, ore processing, waste-water processing, paints, and automobile batteries.
Production of Ammonia:
- The production of ammonia began with the necessities of war. Because ammonia was the precursor for making explosives during World War 1. Nowadays it is used in fertilizer.
- The ammonia synthesis process (also called the Haber-Bosch process) was developed by German chemists Fritz Haber and Carl Bosch. Haber was strongly in favor of the use of chemicals during the war. He developed more lethal gases such as phosgene, chlorine gas, etc. He was the first to use chlorine gas in 1915 at the battle of Ypres. Later on, he was abandoned by his own country.
- In 1913, BASF (a German company) established a plant for making ammonia at the rate of 30 metric tons per day.
Process Description (Haber-Bosch):
Reactor:
Compressed N2 & H2 are sent to the reactor, where the reaction takes place under high pressure and temperature conditions in the presence of an iron catalyst.

Cooling Chamber:
A mixture of gases from the reactor goes to the cooling chamber unit, where liquid nitrogen is withdrawn at the bottom and N2 & H2 are recycled.
Another example is the discovery of Penicillin and its demand for mass production.

The Present-day Chemical Industry:
Chemical engineering finds its application in the solution of the essential needs of mankind.
Food and Nourishment:
Chemical engineers play an important role in increasing the production and processing of food and in the production of fertilizers, insecticides, herbicides, etc.
Clothing and Housing:
Rapidly decreasing natural resources necessitate the introduction of new and better materials for clothing and housing such as synthetic fibers, plastics, polymer, and plywood.
Health Contribution:
Industrial production of drugs and pharmaceuticals.
Energy sector:
The petroleum and petrochemical industries now employ half of the world’s chemical engineers. Petroleum is not unlimited. Chemical engineers are engaged in the utilization of biomass, solar energy, and wind energy.
Communication:
Paper, pencils, printing inks, records, photographic films, magnetic tapes, and disks – all involve the service of chemical engineers in their production stages.
Utilization of natural resources:
Chemical engineer play an important role in the processing and conversion of raw materials into objects needed for daily life.
Protection of the environment:
Harmful and toxic emissions occur during manufacturing processes. It is a chemical engineer’s task to devise methods and equipment to control the release of harmful substances which damage the environment.
Major Chemical Industries:
| Industry | Products | End-use |
|---|---|---|
| Inorganic Chemicals | Sulfuric acid (H2SO4) | Fertilizers, petroleum refining, metal processing, explosives |
| Nitric acid (HNO3) | Explosives, fertilizers | |
| Sodium hydroxide (NaOH) | Soap, Rayon, and film processing industries, cleaners, metal processing Rayon Rayon | |
| Organic Chemicals | Acetic anhydride (C4H6O3) | Rayon, resins, plastics Resin Resin |
Ethylene glycol (C2H6O2) | Antifreeze, dynamite, synthetic fibers Antifreeze Coolant Antifreeze Coolant | |
Formaldehyde (CH2O) | Plastics | |
| Methanol (CH3OH) | Formaldehyde manufacture, antifreeze, solvent | |
| Petroleum & petrochemicals | Gasoline | Motor fuels |
Kerosene  | Jet fuel, domestic fuel | |
| Oils | Lubricating, heating | |
| Ammonia (NH3) | Fertilizers | |
| Ethyl alcohol (C2H5OH) | Acetaldehyde (CH3CHO), solvent | |
Styrene (C8H8)  | Synthetic rubber and plastics | |
| Pulp & paper | Paper, cardboard, fiberboard | Books, records, newspapers, building materials |
| Pigment & paint | Zinc oxide (ZnO2), titanium oxide (TiO2), carbon black | Pigments for paints, ink, plastic, rubber |
| Rubber | Natural rubber, synthetic rubber | Automobiles tires, sheeting, footwear, electrical insulations |
| Minerals | Glass, ceramic, cement | Windows, containers, bricks, pipes, highway |
| Cleansing agents | Soaps, detergents | Household and industrial cleaning |
| Biochemicals | Pharmaceuticals and drugs | Health and medicinal applications |
| Fermentation products | Beverage | |
| Metals | Steel, copper, aluminum, zirconium | Building materials, machinery |
| Uranium | Nuclear fuel |
Functions of a Chemical Engineer:
- Conduct research to develop new and improved manufacturing processes.
- Plant design (construction/operation/product supervision).
- Establish safety procedures for those working with dangerous chemicals.
- Design and plan the layout of the equipment.
- Conduct tests and monitor the performance of processes throughout production.
- Troubleshoot problems with manufacturing processes.
- Estimate production costs for management.
A Well-known Person in Chemical History:

George Edward Davis
The founding father of the discipline of chemical engineering. He was the first one to start lecturing about unit operations at the University of Manchester and wrote the first Handbook of Chemical Engineering.

Fritz Haber
He invented the Haber-Bosch process, a method used in industry to synthesize ammonia (used in fertilizers and explosives) from nitrogen gas and hydrogen gas.

Thomas Chilton & Allan Colburn
Co-founder of modern chemical engineering. They worked together on heat momentum and mass transfer. They developed the “Chilton-Colburn analogy” (widely used in mass and heat transfer courses).

Dr. Elmer L. Gaden
Father of Biochemical Engineering. He introduced chemical engineering principles into biological science while studying at Columbia University. Due to his contributions, it is possible to produce antibiotics, enzymes, and bio-molecules.

Anthony Francis Lucas
Father of Petroleum Engineering. He was an expert at drilling for oil. His development enabled the fossil fuel-based economy.
Dr. Yuan-Cheng Fung
An American scientist, and the founder of tissue engineering and biomechanics.

Dr. Robert Samuel Langer
An American chemical engineer, scientist, entrepreneur, inventor, and an Institute Professor at MIT. He is the father of tissue engineering and drug delivery.

Xi Jinping
He is the General Secretary of the Communist Party of China, the President of the People’s Republic of China, and the Chairman of the Central Military Commission.

Dr. H. L. Roy
He introduced chemical engineering to the curriculum of Bengal National College (presently known as Jadavpur University).

Dr. Raghunath Anant Mashelkar
He is a leading architect of India’s science and technology policies. Former Director-General of the Council of Scientific & Industrial Research, CISR.

Man Mohan Sharma
First Indian chemical engineer to be elected as a Fellow of Royal Society, UK.

Mukesh Ambani
Chairman and Managing Director of Reliance Industries. He received his BE degree in Chemical Engineering from the Institute of Chemical Technology, Matunga, Mumbai.

Padmasree Warrior
She is the former CEO of NIO U.S. She held senior executive positions in Motorola and Cisco. As of 2014, she is listed as the 71st most powerful woman in the world by Forbes.

Harsha Bhogle
He studied chemical engineering at Osmania University. He is an Indian cricket commentator and journalist.
Important questions and answers
Question 1: In the contact process, oxidation of SO2 is carried out at a high temperature and low pressure. However, low temperature and high pressure could have resulted in more yield (more conversion of SO2 into SO3). Why high P and low T conditions are not employed?
Question 2: The formation of ammonia is more favorable at room temperature. Why high temperature and pressure are used during the synthesis of ammonia?
