Differentiate between steady-state and equilibrium state
In this post, we will explore the fundamental differences between steady-state and equilibrium states in thermodynamic systems.
Steady State
In a steady-state process, the system’s properties (state variable such as T, P, density, concentration, etc.) remain constant with time at any given location, but these variables may vary across different locations within the system.
\mathrm{\frac{\partial T}{\partial t}=0}
- Even though matter or energy may be flowing through the system, its overall properties do not change with time.
- Steady-state is a kinetic concept and does not imply that the system is balanced or at rest.
- It only indicates that the rates of input and output are equal, resulting in no net change over time.
- For example, in a continuous chemical reactor, the concentrations of reactants and products remain constant over time, but chemical reactions are continuously taking place.
Equilibrium State
It refers to a state where there are no unbalanced potentials/gradients or driving forces within a system.
- The equilibrium state is a thermodynamic concept where the system has no driving forces for change.
- All gradients, such as chemical potential, temperature, or pressure differences, are zero.
- At equilibrium, there is no net energy or matter exchange within the system or between the system and its surroundings.
Case 1: Metal Bar in a Room (Equilibrium State)
Consider a metal bar placed in a room where both the room and the bar are at 25°C.

- Since there is no temperature difference between the bar and its surroundings, there is no heat transfer.
- The system is in thermal equilibrium, and all gradients (e.g., temperature, pressure) are zero.
- Thus, the system is in both a steady state and an equilibrium state.
Case 2: Metal Bar Being Heated (Steady State but Not Equilibrium)
Now, heat the metal bar at a constant heating rate.
- The lower portion in contact with the heat source will be at a higher temperature than the upper portion, creating a temperature gradient within the bar.
- Heat flows from the hot end to the cooler end.
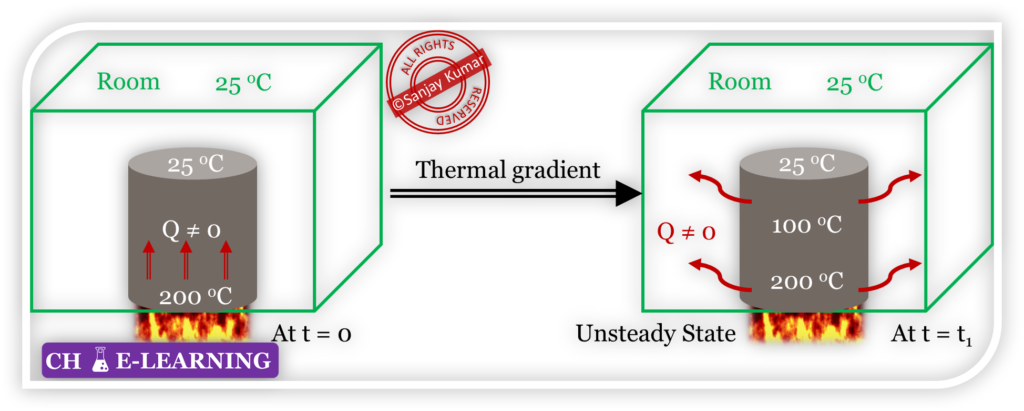
- Also, there is a temperature gradient between the metal bar and the room. At the same time, heat is also being lost from the bar to the surroundings.

- Over time, if the rate of heat input from the source matches the rate of heat loss to the surroundings.
- Then, the temperature of bar will be invariant with time at any location but it may vary with location, indicating the system has reacted in a steady state.
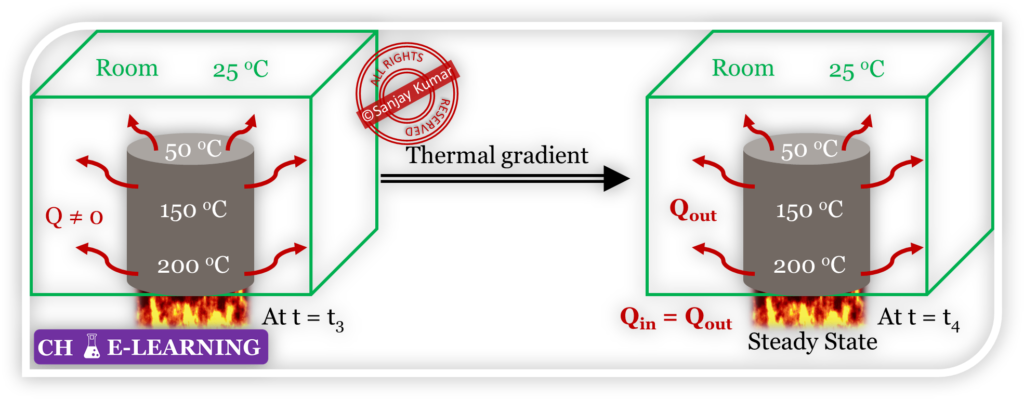
- However, the bar is not in thermal equilibrium because there is a temperature gradient between the bar and its surroundings, and heat is continuously flowing.
Conclusions
- If a system is in an equilibrium state, it is also in a steady state, since no changes occur with time.
- However, a system in a steady state is not necessarily in equilibrium.
- In the steady state, there can still be ongoing processes (e.g., heat or mass transfer), while in equilibrium, all driving forces are balanced, and such processes cease.

Your writing has a way of resonating with me on a deep level. It’s clear that you put a lot of thought and effort into each piece, and it certainly doesn’t go unnoticed.