Problem 11.1: For using brackish water for irrigation, it is to be desalinated by evaporation. A fraction of the feed water is sent through an evaporator and the other part is bypassed. The fraction bypassed and the pure water leaving the evaporator are mixed together to give the desalinated water of the desired quality. If the feed water has 500 ppm (parts per million) of salt, and the salt content in the water used for irrigation is to be limited to a maximum of 50 ppm, determine the fraction of the feed water bypassed.
Problem 11.2: It is desired that the concentration of i-pentane in the feed to a natural gasoline plant should not be more than 10 % by mole. This is prepared by passing a fraction of a hydrocarbon feed stream made up of n-pentane and i-pentane in the mole ratio 4:1 through an i-pentane recovery unit as a side stream. The stream leaving this tower is free of i-pentane. It is mixed with the fraction of the original mixture that is not passed through the recovery tower to get the feedstock to the natural gasoline plant. What percent of the original hydrocarbon mixture should be treated to remove the isopentane?
Problem 11.3: A process stream contains 4 % (weight) salt and the rest water. This is prepared by passing a part of a pure water stream through a saturator containing the salt. The solution leaving the saturator containing 20 % salt is mixed with the pure water bypassed to get the process stream. What fraction of the pure water available is to be passed through the saturator?
Problem 11.4: The maximum allowable limit of impurities in the effluent from a processing unit is 100 ppm, whereas the discharge from the plant contains 500 ppm impurities. To reduce the level of impurities to the allowable limit, a fraction of the effluent is sent through a treatment plant which brings down the impurities to 10 ppm and mixes the treated effluent with the fraction of the effluent that is bypassed. Determine the fraction of the effluent that is bypassed.
Problem 11.5: Air at 313 K saturated with water vapor is dehumidified by cooling and condensation of water vapor at 286K and by consequent condensation of water vapor. Air leaving the dehumidifier saturated at 286 K is mixed with a part of the original air which is bypassed through the dehumidifier. The resulting air stream is reheated to 313 K. It is desired that the final air contains water vapor not more than 0.02 kg/kg of dry air. Calculate: (a) The mass of dry air bypassed (kg) per each kg of dry air sent through the dehumidifier (b) The mass of water vapor condensed (kg) in the dehumidifier per 100 m3 of air sent through it (c) The volume of final air obtained per 100 m3 of air passed through the dehumidifier.
The total pressure is atmospheric and the vapor pressures of water are 1.5 kPa at 286 K and 7.5 kPa at 313 K.
Problem 11.6: In a process for concentrating 1000 kg of freshly extracted orange juice containing 15 % solids, the juice is strained, yielding 750 kg of strained juice and 250 kg of pulpy juice. The strained juice is concentrated in a vacuum evaporator to give an evaporated juice of 60 % solids. The 250 kg of pulpy juice is bypassed around the evaporator and mixed with the evaporated juice in a mixer to improve the flavor. The final concentrated juice contains 40 % solids. Calculate: (a) The mass of final concentrated juice (kg) (b) The concentration of solids in the strained juice (c) The concentration of solids in the pulpy juice.
Problem 11.7: Dry salt is to be produced at a rate of 20000 kg/h by evaporating water from a feed containing 20 % NaCl. The brine (27 % NaCl) leaving the evaporator may either be recycled to the evaporator or may be discarded. If the brine produced is 25 % of the weight of dry salt, calculate: (a) The feed rate if the brine is recycled (b) The percent excess of the feed rate if the brine is discarded over that required when the brine is recycled.
Problem 11.8: Ten thousand kilograms of an equimolar mixture of benzene and toluene is subjected to fractionation (distillation) so as to produce a distillate having a composition of 95 % benzene and the bottom product with a composition of 96 % toluene. The column is provided with a condenser in which the vapor product leaving the top is totally condensed. It was found that the vapor flow rate was 8000 kg/h. A part of the condensate is recycled to the column as reflux and the other part is removed as the distillate product. The vapor, reflux, and the distillate have the same composition. All compositions are on a mole basis. Determine the reflux ratio of the column if it is defined as the quantity of condensate recycled to the quantity withdrawn as a distillate product.
Problem 11.9: For the preparation of potassium nitrate, 10000 kg/h of a 20 % KNO3 solution is mixed with a recycle stream and sent to an evaporator. The rate of evaporation is 1.25 times the rate of the introduction of the recycle stream. The concentrated solution leaving the evaporator contains 50 % KNO3. This is admitted to the crystallizer which yields crystals containing 4 % water. At the crystallization temperature, the solubility is 60 kg/ 100 kg of water. The major part of the mother liquor leaving the evaporator is returned to the evaporator as recycle. Calculate: (a) The concentration of KNO3 in the stream entering the evaporator (b) kg/h of recycle stream (c) The rate of production of crystals.
Problem 11.10: In a wood drier, the hot air must contain at least 2.5 % water to prevent the wood from drying too rapidly and splitting or warping. The original fresh air fed contains 1 % water. Wood is dried from 25 % water to 5 % water. The wet air leaving the drier contains 4 % water. Calculate the amount of wet air that must be returned to the drier if 1000 kg per hour of wet wood is dried. All moisture contents are on a wet basis.
Problem 11.11: The final purification stage in the preparation of vitamins from natural sources requires centrifuging and continuous filtration, as depicted in Figure.
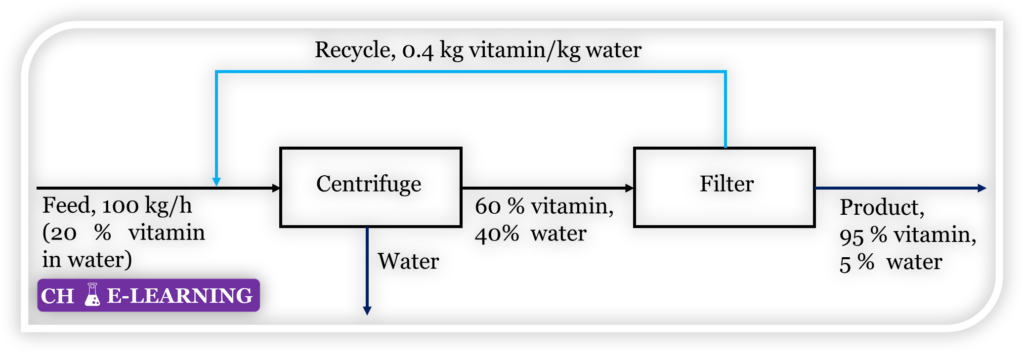
Determine the flow rate of the recycle stream in kg/h.
Problem 11.12: Fresh atmospheric air mixed with recycled air is heated to 349 K and admitted to a drier at 101.3 kPa at a rate of 100 m3/min. The relative humidity of air entering the drier is 7.5 %. Atmospheric air is at 302 K and has a relative humidity of 30 %. The air leaves the drier at 306 K and 90 % relative humidity, a portion of which is recycled and the other portion is discarded. The vapor pressures of water are 4 kPa at 302 K, 5 kPa at 306 K, and 40 kPa at 349 K. Calculate: (a) The ratio of wet recycle stream to wet fresh stream (b) The mass of dry air (kg) to be fed to the drier to remove 1 kg water in the drier (c) The rate at which water is removed in kg/min.
Problem 11.13: Wet solid containing 75 % water is mixed with recycled dry solid to reduce the water content to 50 % before being admitted into the granulator. The solid leaving the granulator is fed to a drier where it is brought into contact with dry air initially containing 0.3 % water by weight. In the drier, the air picks up moisture and leaves with a moisture content of 6 %. The solids leaving the drier contain 15 % water. A portion of this solid is recycled.

For 5000 kg/h of wet solid sent to the granulator as fresh feed, determine: (a) The amount of solid recycled (b) The circulation rate of air in the drier on a dry basis.
Problem 11.14: Fresh air with a dew point of 290 K is used in an adiabatic dryer which recycles a portion of the wet air. The combined mixture of fresh air and recycled air is passed through a heater which raises its temperature to 365 K before being admitted to the dryer. The wet bulb temperature of the air entering the dryer is 310 K and the air leaving the dryer is 95 % saturated. Calculate: (a) The fraction of waste humid air recycled (b) The kilograms of water evaporated from the wet feed per 100 m3 of hot air entering the dryer at 101.3 kPa.
Problem 11.15: Wet air at 365 K and 101.3 kPa has a relative humidity of 15 %. It is to be dried at the rate of 500 m3/h by using sulphuric acid to absorb the moisture from air in a packed tower. The dried air will leave the tower at 327 K and 101.3 kPa and its relative humidity will be 10 %. The spent acid (65 % H2SO4) is partly withdrawn and partly recirculated after making up with fresh 98 % H2SO4 so that the acid entering the tower has a concentration of 75 % H2SO4. Calculate: (a) Cubic meters of wet air leaving the scrubber per hour (b) Kilograms of make-up acid per hour (c) Kilograms of acid entering the tower per hour.
Vapor pressure of water at 327 K = 15 kPa and at 365 K = 75 kPa.
Problem 11.16: A feed consisting of 30 % benzene, 40 % toluene, and 30 % xylene is separated into a distillate containing 93 % benzene 5.5 % toluene, and 1.5 % xylene and a bottom product containing 2.0 % benzene. All percentages are on a mole basis. The reflux ratio is 3.0 and the feed rate is 100 kmol/h. Calculate: (a) Composition of the residue (b) Masses of distillate and residue (c) Recovery of xylene plus toluene in the residue (d) Mass of vapor condensed in the condenser.
Problem 11.17: It is desired to produce 1000 kg/h of Na3PO4.12H2O crystals from a feed solution containing 6 % (weight) Na3PO4 and traces of impurity. The original solution is first evaporated in an evaporator to a 40 % (weight) Na3PO4 solution and then cooled to 293 K in a crystallizer, where the hydrated crystals and mother liquor solution are removed. One out of every 10 kg of mother liquor is discarded to waste to get rid of the impurities, and the remaining mother liquor is recycled to the evaporator. The solubility of Na3PO4 at 293 K is 9.9 % (weight). Calculate: (a) Feed rate (kg/h) (b) Rate of evaporation of water (kg/h) (c) Rate at which the mother liquor is recycled (kg/h) (d) Concentration of the solution entering the evaporator.
Problem 11.18: A solution containing 10 % NaCl, 5 % KCl, and 85 % water is mixed with a recycle stream and sent to the evaporator. In the evaporator is removed as vapor and NaCl is removed as crystals. The concentrated solution leaving the evaporator (NaCl = 17.0 %, KC1 = 22 %, H2O = 61 %) is sent to a crystallizer from which KCl and NaCl crystals are removed. The mother liquor leaving the crystallizer (NaCl = 15.86 %, H2O = 84.14 %) is sent back to the evaporator. Calculate the quantity and composition of the combined stream entering the evaporator if the feed rate is 20000 kg/h.
Problem 11.19: A distillation column separates 10000 kg/h of a 55 % benzene and 45 % toluene mixture. The product recovered from the condenser at the top of the column contains 97 % benzene and the bottoms of the column contain 98 % toluene. The vapor stream entering the condenser from the top of the column is 9000 kg/h. A portion of the product is returned to the column as reflux, and the rest is withdrawn for use elsewhere. Assume that the composition of the streams at the top of the column, the product withdrawn, and the liquid reflux are identical. All compositions are on a weight basis. Find the ratio of the amount refluxed to the product withdrawn.
Problem 11.20: A solid material containing 12 % moisture is dried so that it contains 5 % water by blowing fresh warm air mixed with recycled air over the solid in the drier. The inlet fresh air has a humidity of 0.01 kg of water/kg of dry air, the air from the drier that is recycled has a humidity of 0.1 kg of water/kg of dry air, and the mixed air to the drier 0.04 kg of water/kg of dry air. For a feed of 1000 kg solid/h fed to the drier, calculate: (a) kg/h of dried product (b) kg of dry air/h in the fresh air (c) kg of dry air/h in the recycled air.
Problem 11.21: Fresh air containing 0.01 kg of water/kg of dry air is mixed with recycled air containing 0.15 kg of water/kg of dry air and is blown over a wet solid in a drier. In a certain operation, the wet solid contains 15 % (weight) moisture and it is to be dried to a final moisture content of 5 % by evaporating moisture into the air blown over it. The fresh air and recycled air are mixed in such proportions that the air blown over the solid contains 0.03 kg of moisture/kg of dry air. A part of the air leaving the drier which contains 0.1 kg of water is recycled. For 100 kg of wet material charged, determine: (a) The ratio of dry air in the recycled air to that in the fresh air (b) The quantity of dry air in the fresh air feed (c) The amount of dry air recycled.
Problem 11.22: 11.22 A solution containing 10 % NaCl, 3 % KCl, and 87 % water is fed to the process shown in Figure at the rate of 20000 kg/h.
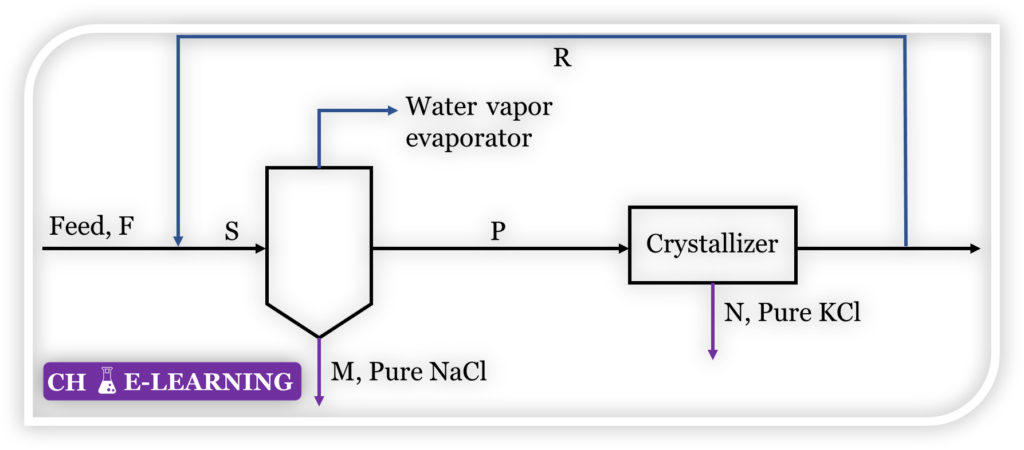
The composition of the evaporator product P is NaCl = 16.8, KCl = 21.6, H2O = 61.6. Calculate: (a) Flow rate of stream P (b) Flow rate of stream R (c) Rate of evaporation (d) Quantity and composition of the stream entering the evaporator.
Problem 11.23: A wet organic pigment containing 40 % CCl4 is to be dried to 5 %. The drier can handle only 200 kg/h of dry solid. The air used for drying is obtained by mixing fresh air with recycled air. The fresh air is essentially free of CC14 and it enters the dryer at 373 K and 101.3 kPa with a dew point 288 K for CCl4. The air leaving the drier has a CCl4 dew point of 298 K. Calculate: (a) The fresh air to be supplied to the drier (kg/h) (b) The recycle ratio, CC14-free basis.
The vapor pressure of CCl4 at 288 K = 7.60 kPa and at 298 K 15.3 kPa.
Problem 11.24: Cloth is dried in a stenter (hot-air dryer) in a textile mill. In this machine, fresh air first mixes with recirculated air. The mixture then passes through a heater. Hot air is jetted over the cloth to evaporate the mixture. A major portion of the jetted air is recirculated while the remaining small portion is exhausted. The operation is shown schematically in Figure.
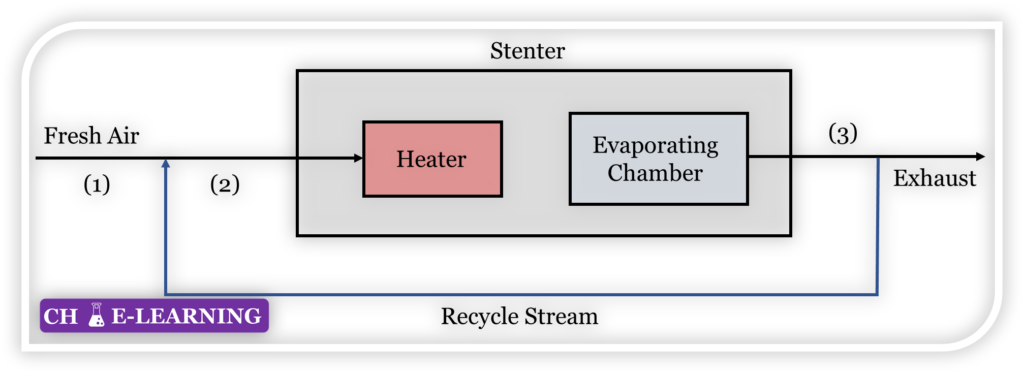
Test data on a particular run of a stenter are given below:
Cloth details: width: 1.1 m, density: 0.085 kg/m3 (dry basis), speed: 60 m/min, inlet moisture: 83 % (dry basis), outlet moisture: 10 % (dry basis).
Air conditions: Moisture of air at point (1): 0.02 kg/kg of dry air, Moisture of air at point (2): 0.09 kg/kg of dry air, Moisture of air at (3): 0.10 kg/kg of dry air.
Calculate: (a) The rate of evaporation in the stenter (b) The mass flow rate of fresh air (c) The volumetric flow rate of fresh air (m3/min), if it is supplied at 300 K and 100 kPa (d) The mass flow rate of recirculating dry air.
Problem 11.25: In a process producing KNO3 salt, 1000 kg/h of a feed solution containing 10 % KNO3 is fed to an evaporator which evaporates some water to produce a 50 % KNO3 solution. This is then fed to a crystalliser, where crystals containing 95 % KNO3 are removed. The saturated solution containing 35 % KNO3 is recycled to the evaporator. Calculate: (a) The amount of recycle stream (kg/h) (b) The amount of crystals (kg/h) (c) The quantity of water evaporated (kg/h).
Problem 11.26: A schematic diagram of a process is shown in Figure.

The composition of various streams is as follows:
| Component | Mol % F | Mol % I | Mol % J | Mol % K |
| A | 15 | 4 | 7 | 60 |
| B | 20 | 29.4 | 27 | 6.5 |
| C | 50 | 54 | 44.4 | 16.5 |
| D | 15 | 12.6 | 21.6 | 17 |
Given that 80 % of A in J is present in K and the ratio of F to P is 1.63, calculate: (a) R/J (b) Composition of P.
Problem 11.27: A schematic flow sheet of a process is shown in Figure.

The following table gives the composition of various streams in mole %.
| Component | Mol % F | Mol % L | Mol % M | Mol % N |
| A | 20 | 30 | 27.6 | 6 |
| B | 50 | 50 | 40.4 | 14 |
| C | 15 | 5 | 8 | 60 |
| D | 15 | 15 | 24 | 20 |
It is known that stream N contains 75 % of C present in stream M. The ratio of F/P is 5:3 on mole basis. Calculate: (a) R/M (b) Composition of R (c) Composition of P.
Problem 11.28: A boiler in a process plant is supplied with feed water containing 250 ppm solids. Steam produced does not carry any solid particles. If the solids content in the boiler at any time should not exceed 1500 ppm, calculate the percentage boiler blowdown based on the quantity of steam produced when (a) The entire feed to the boiler is fresh feed water (b) 50 % of the feed entering the boiler is constituted by the condensate recycled from the boiler.
Problem 11.29: A boiler feed water contains 2000 ppm dissolved solids. The permissible limit of dissolved solids in the water in the boiler is 1 part per 100 parts. What percent of the feed water is to be blown down?
Problem 11.30: High-pressure steam generated in a plant is sent to the power generation unit, 50 % of which is further utilized in the plant as process stream and the remaining 50 % is sent back to the boiler as condensate. The condensate carries with it dissolved solids to the extent of 50 ppm. To keep the solid content in the boiler water within 1500 ppm, a part of the boiler water is blown down continuously. To make up for the blowdown as well as for the water lost as the process steam, feed water containing 450 ppm solids is fed to the boiler. Calculate the feed to blowdown ratio.
Problem 11.31: Seawater is desalinated by reverse osmosis, as shown in Figure. All compositions are on a mass basis. Calculate R/E.

Problem 11.32: Potable water containing not more than 500 ppm dissolved salt is made by desalinization through reverse osmosis of seawater which contains 3.0 % salt. Fresh seawater is admitted at a rate of 1000 kg/h. Potable water is withdrawn from the reverse osmosis cell as a product while a fraction of the brine that leaves the cell and containing 5.25 % salt is recycled. The concentration of salt in the stream entering the cell after mixing the recycle stream with fresh seawater is 4.0 %. Determine: (a) The rate at which brine is removed from the plant (kg/h) (b) The rate at which potable water is produced (kg/h) (c) The fraction of the brine leaving the cell that is recycled.
Problem 11.33: Methanol is converted to formaldehyde according to:
2CH_3OH+O_2\rightarrow2HCHO+2H_2O CH_3OH\rightarrow HCHO+H_2A mixture containing 80 % methanol and 20 % oxygen is supplied as fresh feed in a formalin plant. The conversion of methanol per pass is 50 % and all the oxygen is converted. The exit stream from the reactor contains unconverted methanol, hydrogen, water and formaldehyde. All the formaldehyde and water are removed from the separator. The hydrogen is recovered from the recycle stream before it is sent to the reactor. For a basis of 100 kmol methanol entering as fresh feed, determine: (a) The mass of formaldehyde produced (kg) (b) The mass of hydrogen produced (kg) (c) The mass of methanol recycled (kg).
Problem 11.34: The process schematic of a propane dehydrogenation plant is shown below in Figure.
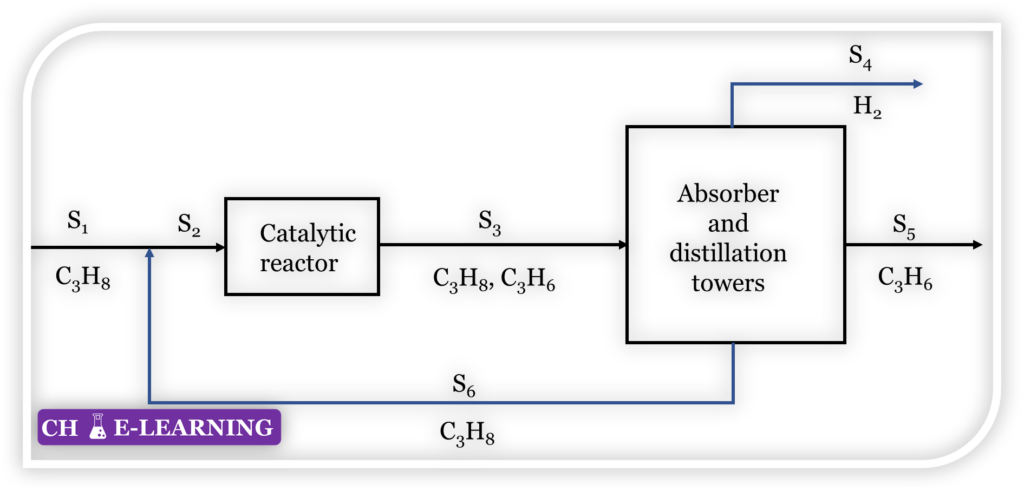
It is desired to set up a simplified version of the material balance for this plant. Assume that the only reaction is the dehydrogenation of propane to propylene; there are no side reactions. The yield of propylene per pass is 30 %. Assume that the amount of carbon formed on the catalyst is negligible. The product flow rate (stream S5) is 60 kmol/h. Calculate the flow rates of all the other streams. Notice that all streams except stream S3 are pure.
Problem 11.35: Methanol is produced by the reaction of CO with H2.
CO+2H_2\rightarrow CH_3OHOnly 15 % of carbon monoxide entering the reactor is converted to methanol. The methanol formed is condensed and recovered completely. The unreacted CO and H2 are recycled back to the reactor. The feed will contain H2 and CO in the ratio 2:1. For 3200 kg/h of methanol produced, calculate (a) The amount of fresh feed (kmol/h) (b) The amount of recycle gas (kmol/h).
Problem 11.36: It is proposed to produce acetaldehyde by oxidation of ethanol in the gas phase.
C_2H_5OH(g)+\frac12O_2(g)\rightarrow CH_3CHO(g)+H_2O(g)the ratio of air to ethanol in the fresh feed (before it is mixed with the recycle stream) is 10 to 1 by mole. The conversion of ethanol on a single pass through the reactor is 25 %. The unreacted ethanol is completely separated from the reaction products and recycled. (a) What is the ratio of the recycle stream to the fresh feed stream? (b) What is the composition of the outlet stream from the reactor in mass percent and mole percent?
Problem 11.37: Glucose (C6H12O6) is enzymatically converted to fructose (C6H12O6) in a fixed-bed reactor. Only a portion of the exit stream from the reactor is removed as a product, the other portion being recycled to the reactor. The ratio of the moles of product to the recycle stream is 8.5. The recycle stream is mixed with the fresh feed and the combined stream containing 5 % fructose is admitted to the reactor. The fresh feed is a 6 0% glucose solution in water. What is the percent conversion of glucose to fructose on one pass through the reactor?
Problem 11.38: Limestone containing 95 weight% CaCO3 and 5 % SiO2 is calcined in a kiln.
CaCO_3\rightarrow CaO+CO_2The heat requirement in the kiln is met by burning coke which is essentially carbon in a furnace and by admitting the hot flue gases into the kiln. The flue gas analysis at the furnace exit shows 8 % CO2. This is mixed with a fraction of the gases leaving the kiln so that CO2 content at the kiln inlet is 10 % CO2. If the gases leaving the kiln analyzed 12 % CO2, determine for 100 kg coke burned,
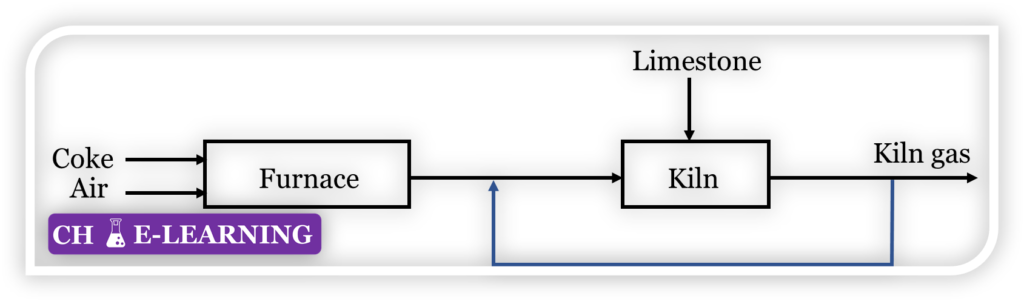
(a) The mass of lime produced (kg) and its percent purity. (b) Recycle ratio (c) Moles of stack gases.
Problem 11.39: Methanol vapor can be converted into formaldehyde by the following reaction scheme:
CH_3OH+\frac12O_2\rightarrow HCHO+H_2O CH_3OH\rightarrow HCHO+H_2The fresh feed to the process is 0.5 k mol/h of O2 and an excess methanol. All of the oxygen reacts in the reactor. Formaldehyde and water are removed from the product stream first, after which hydrogen is removed from the recycled methanol. The recycle flow rate of methanol is 1 kmol/h. The ratio of methanol reacting by decomposition to that by oxidation is 3. Calculate: (a) The fresh feed rate of methanol (b) The single pass conversion of methanol (c) The hydrogen produced.
Problem 11.40: The chlorination of methane to form methyl chloride results in the formation of some polychlorinated methane also. A mixture of methane and chlorine in the mole ratio 10:1 is sent to a reactor. The products analyse 92.2 % CH4, 6.2 % CH3Cl, 1.0 % CH2Cl2, 0.4 % CHCl3, 0.1 % CCl4 on an HCl-free basis. The mixture is scrubbed with water to remove HCl gases. The chlorinated products in the gas leaving the scrubber are condensed and removed from the excess methane which is recycled. Additional chlorine is added to keep the methane: chlorine ratio in the feed to the reactor at 10:1. For a basis of 100 kg of chlorinated products per hour, determine: (a) Moles of methane and chlorine admitted as fresh feed. (b) The mass of methane (kg) recycled per hour (c) The percent excess chlorine in the fresh feed based on conversion to methyl chloride (d) The cubic meters of gas leaving the separator at STP.
Problem 11.41: In an attempt to provide a means of generating NO cheaply, gaseous ammonia is burned with O2. It is desired that oxygen entering the reactor is 20 % in excess of that theoretically required. The reaction is 70 % complete. The nitric oxide is separated from the unreacted NH3, and the latter is recycled, as shown in Figure.
4NH_3+5O_2\rightarrow4NO+6H_2O
Calculate: (a) The moles of ammonia recycled per 100 moles of NO formed (b) The ratio of moles of ammonia to oxygen in the fresh feed.
Problem 11.42: Figure shows the three main steps in the production of vinyl chloride from ethylene-chlorination (A), oxyhydrochlorination (B) and pyrolysis (C).
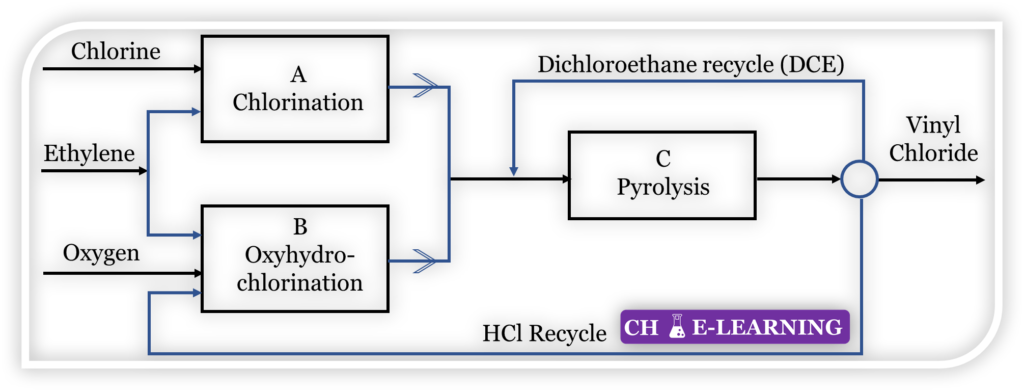
The reactions occurring are: Chlorination: (The yield on ethylene is 98 %)
C_2H_4+Cl_2\rightarrow C_2H_4Cl_2Oxyhydrochlorination: (Yield on ethylene is 95 %, on HCl 90 %)
C_2H_4+2HCl+\frac12O_2\rightarrow C_2H_4Cl_2+H_2OPyrolysis: (Yield on dichloroethylene is 99 %, on HCl 99.5 %)
C_2H_4Cl_2\rightarrow C_2H_3Cl+HClThe HCl from the pyrolysis step is recycled to the oxyhydrochlorination step. The flow of ethylene to the chlorination and oxyhydrochlorination reactors is adjusted so that the production of HCl is in balance with the requirement. The conversion in the pyrolysis reaction is limited to 55 %, and the unreacted dichloroethane is separated and recycled. For the production of 25000 kg/h of vinyl chloride, determine (a) the flow of ethylene to each reactor and (b) the total flow of dichloroethane to the pyrolysis reactor.
Problem 11.43: Methanol is produced by the reaction of CO with H2.
CO+2H_2\rightarrow CH_3OHThe side reaction is
CO++3H_2\rightarrow CH_4+H_2OThe conversion per pass is 15 %. Of this amount, 87.5 % is assumed to react via equation (A) and 12.5 % via equation (B). The stream leaving the reactor passes through a condenser and a separator. The CO and H2 leaving these units are recycled. The ratio of H to CO in the recycle is 2:1. The CH4 leaves as a gas and the liquid mixture of methanol and water passes to a distillation column for the concentration of methanol. The flow diagram is given in Figure.

For 100 moles of fresh feed, determine: (a) Moles of gas recycled (b) Composition of the stream leaving the reactor (c) Moles of methanol produced.
Problem 11.44: In the production of ammonia from hydrogen and nitrogen, the conversion based on either raw material is limited to 15 %. The ammonia produced is condensed and separated. The uncondensed gases are recycled. The argon in the recycle is to be limited to 5 %. If the feed contains 0.2 % argon, calculate the purge required.
Problem 11.45: o-Xylene is oxidized to phthalic anhydride by oxygen according to:
C_8H_10+3O_2\rightarrow C_8H_4O_3+3H_2OA mixture of o-xylene and air containing 6 % xylene is sent to a fixed-bed reactor at 625 K along with a recycle stream coming from the condenser in which the phthalic anhydride and water produced in the reactor are separated and removed. The recycle is provided with a purge and the purge stream analyzed 78 % N2. It is found that for every mole of gas mixture entering as fresh feed, 1.2 moles leave the reactor. If 60 % conversion is achieved in a single pass and 100 kmol of a mixture of xylene and air is admitted as fresh feed, determine: (a) the rate of production of pthalic anhydride (kg/h) and (b) xylene produced (kg/h).
Problem 11.46: A 1:3 nitrogen-hydrogen mixture is fed to a converter resulting in 20 % conversion to ammonia. After complete separation of ammonia, the remaining unconverted gases are recycled to the converter. The initial reaction mixture contains 0.2 % by volume argon. If the limit of argon in the reactor is 5 % by volume of the N2-H2 mixture in the reactor, estimate: (a) Fraction of the recycle that is purged (b) Moles of ammonia produced per 100 mole feed (c) Overall conversion of ammonia.
Problem 11.47: Methanol is made by the reaction of CO with H2 according to the reaction
CO+2H_2\rightarrow CH_3OHThe gas mixture entering the reactor is contaminated by small amounts of methane. The composition of the gas mixture is as follows: CO = 32.5 %, H = 67.3 % and CH4 = 0. 2%
The single-pass conversion of CO in the reactor is only 18 %. The exit stream from the reactor is sent to a separator in which methanol is separated and removed. The unconverted gases are recycled and mixed with the fresh feed before being admitted to the reactor. It is desired that the methane content in the recycle stream is not more than 3.0 %. This is achieved by bleeding off a portion of the recycle stream at the exit of the separator. Calculate per 100 moles of fresh feed (a) The moles of recycle stream (b) The moles of the purge stream (c) Composition of the purge stream (d) Moles of methanol produced.
Problem 11.48: Refer to the Figure given below. Ethylene is oxidized to ethylene oxide by the reaction:
C_2H_4+\frac12O_2\rightarrow CH_2CH_2O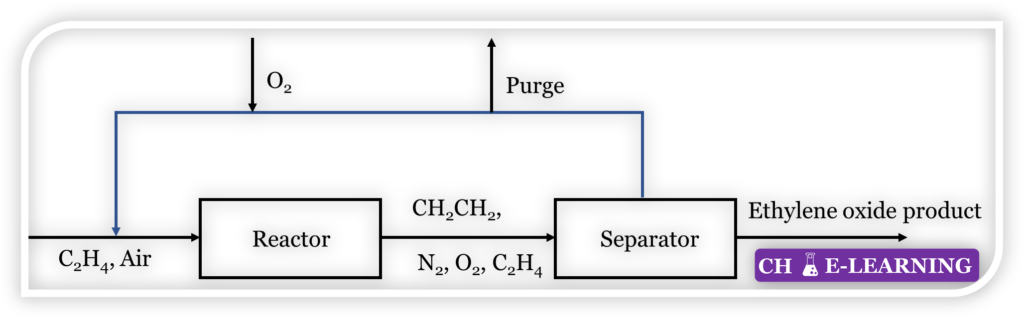
The ethylene to air ratio in the stream entering the reactor is to be 1:10. The conversion of ethylene is 25 % per pass. Ethylene oxide is separated and the unconverted gas is recycled. To maintain the ethylene-air ratio in the reactor, 25 % by volume of the recycle stream is purged off. Also, pure oxygen is supplied in addition to the ethylene- air mixture supplied as fresh feed. For producing 100 kg of ethylene oxide per hour, calculate: (a) Ethylene-air ratio in the fresh feed (b) Moles of gas purged off (c) kg/h of ethylene to be supplied as fresh feed (d) kg/h of pure oxygen supplied.
Problem 11.49: In the Haber process for the manufacture of ammonia, synthesis gas which analyses 75.2 % H2, 24.5 % N2 and 0.3 % Ar is sent to the converter after mixing with recycle stream. The following reaction takes place in the converter:
N_2+3H_2\rightarrow2NH_3To avoid building up of argon in the recycle stream, which will adversely affect the equilibrium conversion after a certain concentration, a small purge is provided in the recycle. The gas mixture entering the converter contains 79.5 % H2. Ammonia is separated from the converter gases in a separator and the unreacted gases are recycled. The gases leaving the separator are free from ammonia and contain 80.0 % H2 and the product ammonia contains no dissolved gas. For a basis of 100 moles of fresh feed, calculate: (a) The moles of the recycle stream (b) The moles of the purge stream (c) The percent conversion of hydrogen per pass.
Problem 11.50: A synthesis gas consisting of 24.3 % N2, 75.2 % H2, and 0.5 % Ar is fed to an ammonia converter mixed with the recycled stream. The single-pass conversion of nitrogen is 10 %. Unconverted gases leaving the reactor is separated and returned to the reactor along with fresh feed. In order to keep the concentration of Ar in the recycled stream below 1 %, a portion of the recycled stream is purged off. For 100 kg of fresh synthesis gas, determine: (a) Moles of recycle stream (b) Moles of purge stream (c) Ammonia produced (kmol).
Problem 11.51: A gas mixture containing nitrogen and hydrogen in the ratio of 1:3 by volume is sent to the ammonia converter where its conversion to NH3 is 25 % complete. The ammonia formed is condensed and separated by passing the gases leaving the converter through a condenser. The unconverted gases are recycled. To prevent the accumulation of argon, a portion of the recycle stream is purged off. The fresh feed contains 0.003 mol argon per mole of N2-H2 mixture, and the level of argon in the reactor should not exceed 0.05 mol per mol of N2-H2 mixture, determine: (a) The fraction of the recycle stream that is purged off (b) The concentration of Ar in the recycle stream (c) Moles of N2-H2 mixture entering the reactor per 100 moles of gas admitted as fresh feed.
Problem 11.52: Benzene is produced from toluene as per the following reaction:
C_7H_8+H_2\rightarrow C_6H_6+CH_4The following side reaction is also found to occur:
2C_7H_8+H_2\rightarrow C_{12}H_{10}+2CH_4The reaction is carried out according to Figure.

It is known that the conversion of toluene per pass is 75 %. And 90 % of the toluene reacted is converted to benzene. For 100 moles of toluene entering the reactor, calculate: (a) Moles of gas purged (b) Composition of the purge stream (c) Moles of makeup hydrogen.
Problem 11.53: N2 and H2 in the ratio of 1:3 constitute the synthesis gas for the manufacture of ammonia. Synthesis gas is obtained by burning excess hydrogen in the air. Since air contains 0.95 % Ar, the gas produced will be contaminated with Ar. The gas is fed to a converter after mixing with the recycle stream. The conversion of the gas to ammonia is only 25 % complete. The unconverted gases are separated from the product ammonia and are recycled. The combined stream consisting of fresh synthesis gas and the recycle stream should not contain Ar above 5 %. To keep the level of Ar below this, a portion of recycle is purged off. For 100 kmol of hydrogen in the fresh feed, determine: (a) The moles of ammonia produced (b) The percent of hydrogen in the fresh feed that is converted to ammonia (c) The moles of gas purged off.
