Why cp is always greater than cv for compressible substances?
In this post, you will learn why specific heat capacity at constant pressure (cp) is always greater than specific heat capacity at constant volume (cv).
- Consider two systems in which heat is supplied at constant pressure and constant volume.
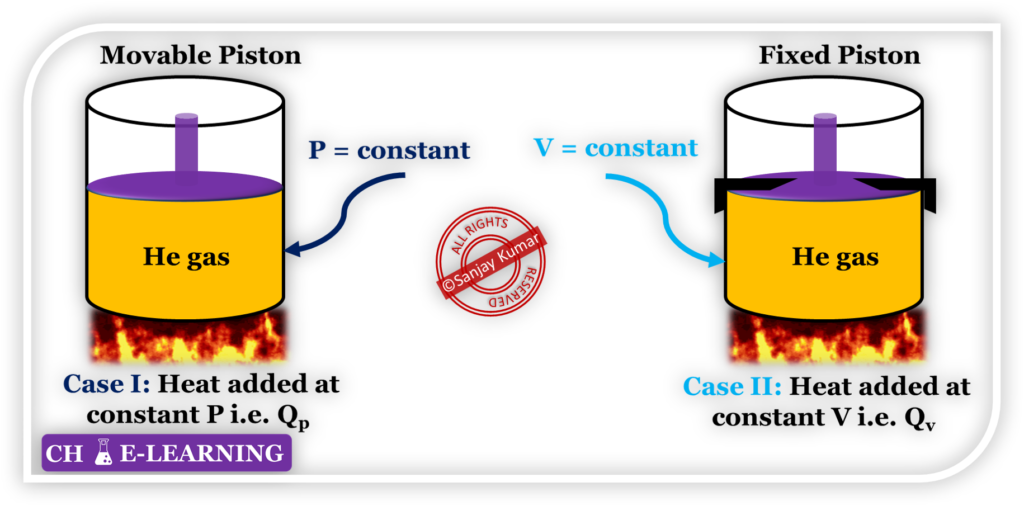
- We want to raise the temperature of the same amount of Helium gas in both systems by the same ∆T.
\mathrm{Q_p=mc_p\triangle T;\;\;Q_v=mc_v\triangle T}
In case 1
As the piston is movable, the system is capable of doing work on the surroundings after getting heat from an external source.
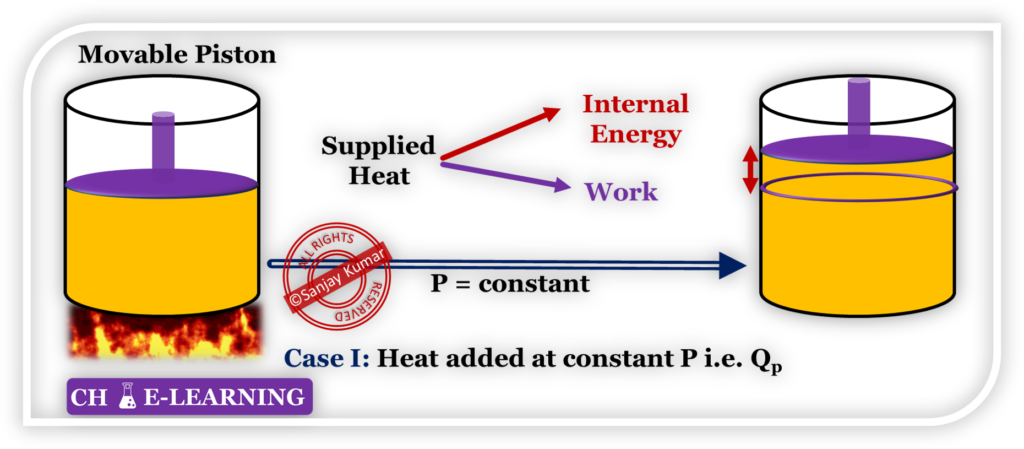
- Therefore, some part of the supplied heat (Qp) will be utilized to increase the internal energy of the system, and the rest part in doing work on the surrounding.
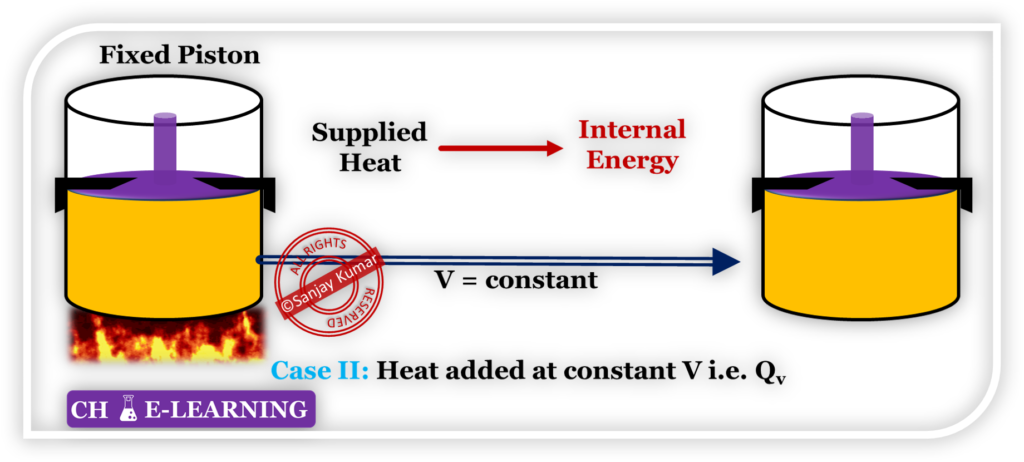
In case II
In this case, the piston is not movable. So all supplied heat (Qv) will be utilized to increase the internal energy of the system.
Critical Thinking
In case 1, a part of the supplied heat is utilized to raise the temperature of the system. In case II, all the supplied heat is utilized to raise the temperature of the system.
- So, more amount of heat has to be supplied at constant P than at constant V to raise the temperature by the same ∆T.
\mathrm{\frac{c_p}{c_v}=\gamma\Rightarrow\gamma>1}

Just wish to say your article is as surprising The clearness in your post is just cool and i could assume youre an expert on this subject Fine with your permission allow me to grab your RSS feed to keep updated with forthcoming post Thanks a million and please keep up the enjoyable work
I do believe all the ideas youve presented for your post They are really convincing and will certainly work Nonetheless the posts are too short for novices May just you please lengthen them a little from subsequent time Thanks for the post