The term biogeochemical cycle is derived from four components:
- Bio → biological (living organisms) or biosphere
- Geo → geological (rocks, air, water, soil)
- Chemical → chemical elements and compounds
- Cycle → circulation or repeated movement
Thus, a biogeochemical cycle refers to the natural pathways through which essential elements (such as carbon, nitrogen, phosphorus, sulfur, and water) circulate between the biotic (living organisms) and abiotic (non-living) components of an ecosystem.
- The circulation of these nutrient elements is also termed nutrient cycling.
- Nutrient cycles are vital for maintaining soil fertility, supporting plant growth, regulating climate, and sustaining the overall balance of ecosystems.
- Without these cycles, ecosystems would quickly deplete the nutrients required for life.
Types of Cycles
Biogeochemical cycles are broadly classified into the following types:
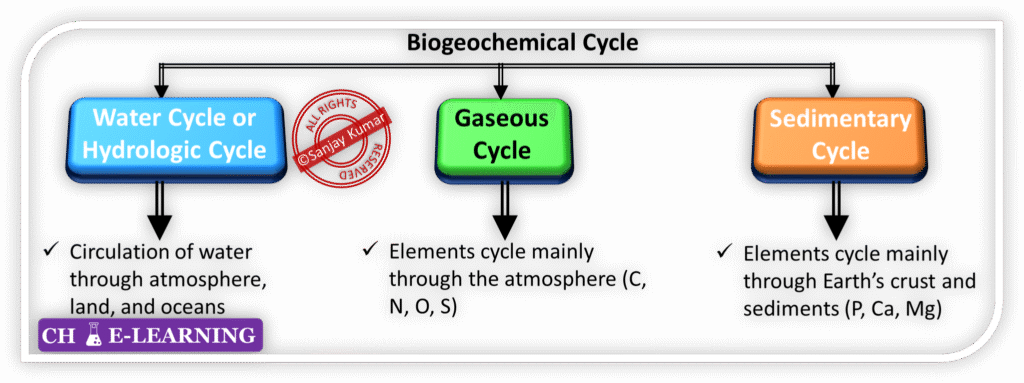
Water (Hydrological) Cycle:
The water cycle is the continuous circulation of water between the atmosphere, lithosphere (land), hydrosphere (oceans, lakes, rivers, and groundwater), and biosphere (living organisms).
- It plays a critical role in regulating Earth’s climate, supporting ecosystems, and sustaining life.
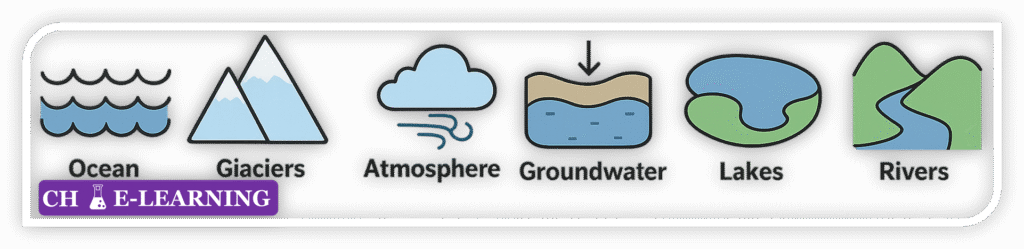
Reservoirs of Water::
- Oceans: The largest reservoir, holding ~96.5% of Earth’s water.
- Glaciers and Ice Caps: Contain ~1.74% of Earth’s water, mostly in frozen form.
- Groundwater: Represents about 1.7% of Earth’s water.
- Freshwater Lakes, Rivers, and Wetlands: Together account for only ~0.013% of total water.
- Atmosphere: Holds ~0.001% of Earth’s water, yet plays a vital role for short-term weather and climate processes.
Forms of Water::
Water exists naturally in three states of matter:
- Solid: Ice, snow, and glaciers.
- Liquid: Rivers, lakes, oceans, and groundwater.
- Gas: Water vapor in the atmosphere.

Processes of Water Circulation::
The water cycle is driven primarily by solar energy and gravity. The major processes are:
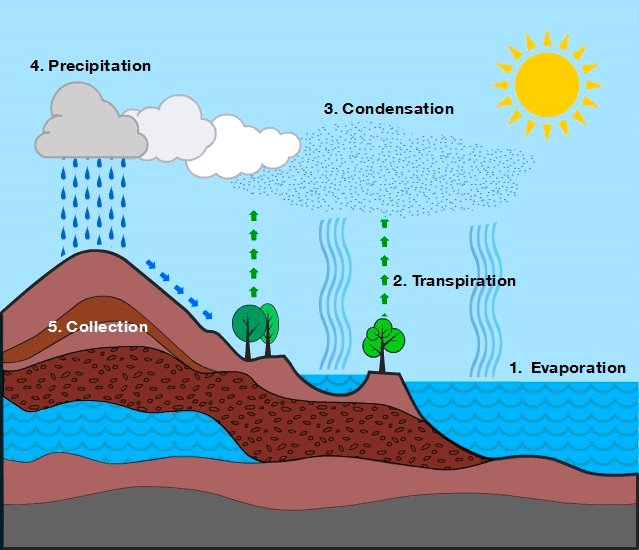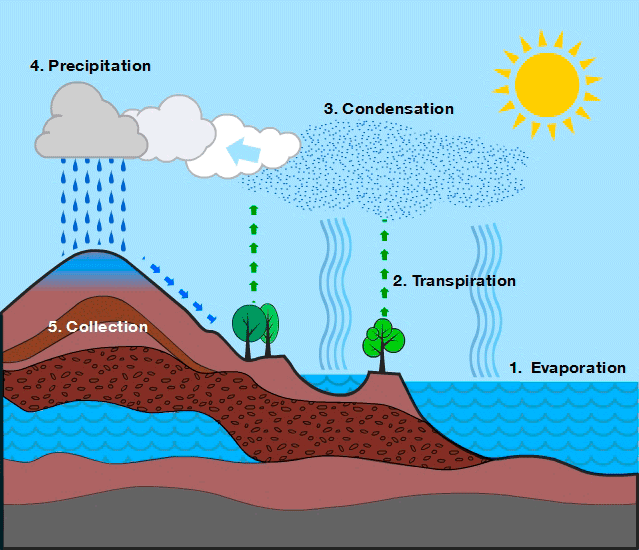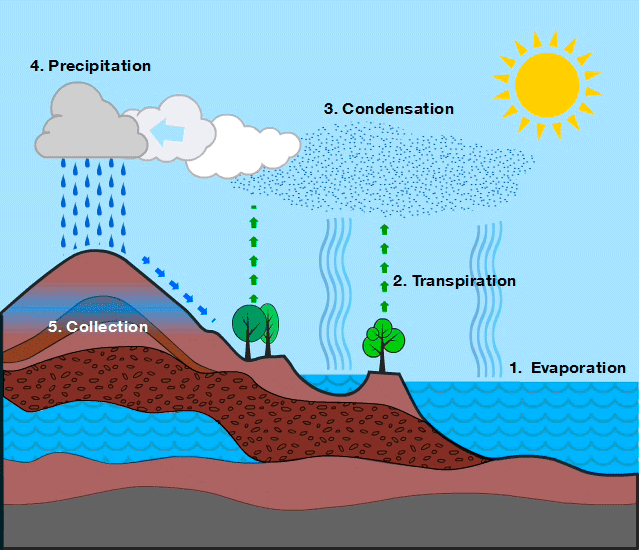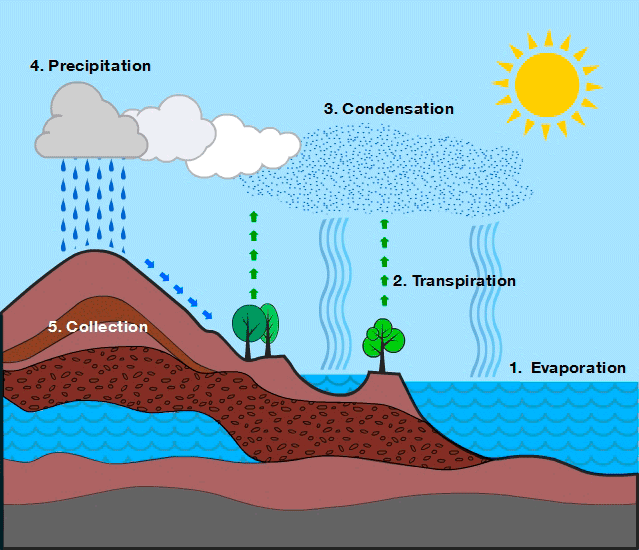
- Evaporation: The process by which liquid water changes into water vapor due to solar heating.
- The Sun heats water in oceans, rivers, and lakes, causing it to transform into vapor.
- Evaporation is the main source of atmospheric water vapor.
- The rate of evaporation depends on factors such as temperature, surface area, wind speed, and humidity.
- Transpiration: The release of water vapor from plant leaves through microscopic pores called stomata.
- Plants absorb water from the soil through their roots, transport it through their tissues, and release it as vapor into the atmosphere.
- Transpiration helps in cooling plants, transporting nutrients, and supporting photosynthesis.
- Together with evaporation, it forms evapotranspiration, a key contributor to atmospheric moisture.
- Condensation: The process by which water vapor in the atmosphere cools and changes back into tiny liquid droplets.
- As air rises and cools, water vapor condenses into tiny droplets.
- These droplets combine with dust and other particles, forming clouds and fog.
- Condensation is essential for precipitation to occur.
- Precipitation: The process by which condensed water in the atmosphere falls to earth’s surface.
- Occurs when cloud droplets or ice crystals become too heavy to remain suspended.
- They fall back to Earth as rain, snow, sleet, or hail.
- Precipitation replenishes rivers, lakes, groundwater, and soil moisture.
- Infiltration: The process by which surface water enters the soil through pore space and rock fractures.
- Some water seeps into the soil and percolates downward due to gravity and capillary action.
- It contributes to groundwater recharge and provides moisture for plant uptake.
- Runoff: The movement of excess water over land surface into rivers, lakes, and oceans.
- Occurs when precipitation exceeds the soil’s infiltration capacity or when surfaces are impermeable.
- Water that does not infiltrate flows across the surface into rivers, lakes, and ultimately the oceans.
- Runoff plays a major role in erosion, sediment transport, and nutrient cycling.
The water cycle is a continuous and dynamic process involving evaporation, transpiration, condensation, precipitation, infiltration, and runoff. It redistributes water across Earth’s reservoirs, ensuring the availability of freshwater and maintaining ecological balance.
Gaseous Cycle:
Gaseous cycles are biogeochemical cycles in which the major reservoirs of elements are located in the atmosphere or hydrosphere. In these cycles, nutrients such as carbon, nitrogen, oxygen, and sulfur circulate rapidly between the atmosphere, water bodies, and living organisms.
- The atmosphere serves as the primary reservoir.
- These cycles are typically faster than sedimentary cycles, ensuring the continuous availability of essential elements to ecosystems.
Oxygen Cycle::
The oxygen cycle refers to the continuous circulation of oxygen among the atmosphere, biosphere, hydrosphere, and lithosphere.
- It plays a vital role in sustaining life, regulating atmospheric composition, and driving biogeochemical processes on Earth.
Reservoir of Oxygen:::
- Lithosphere: The largest reservoir, containing ~99.5% of Earth’s oxygen in the form of oxides (e.g., silica SiO2, alumina Al2O3, iron oxides Fe2O3, carbonates CaCO3, feldspar, dolomite).
- This oxygen exists in a bound form with a very slow exchange rate.
- Atmosphere: Contains ~21% oxygen by volume as free molecular O₂.
- Atmospheric oxygen also occurs in compound such as ozone(O₃), CO₂ and water vapor (H₂O), sulfide oxides (SO2, SO3).
- Hydrosphere: Oceans, lakes, and rivers contain dissolved oxygen, which is essential for aquatic life.
- It also holds dissolved CO2 and carbonic acid (H2CO3).
- Biosphere: Oxygen is stored in living organisms as part of organic molecules such as carbohydrates (C6H12O6), proteins (amino acids), and lipids (fatty acids).
Forms of Oxygen:::
- Free oxygen (O₂): Molecular oxygen available in the atmosphere and dissolved in water bodies.
- Ozone (O₃): Found in the stratosphere (10-50 km), forming the ozone layer that protects Earth from harmful UV radiation.
- Bound oxygen: Found in compounds such as water (H₂O), carbon dioxide (CO₂), and minerals (silicates, carbonates, oxides).
Processes of Oxygen circulation:::
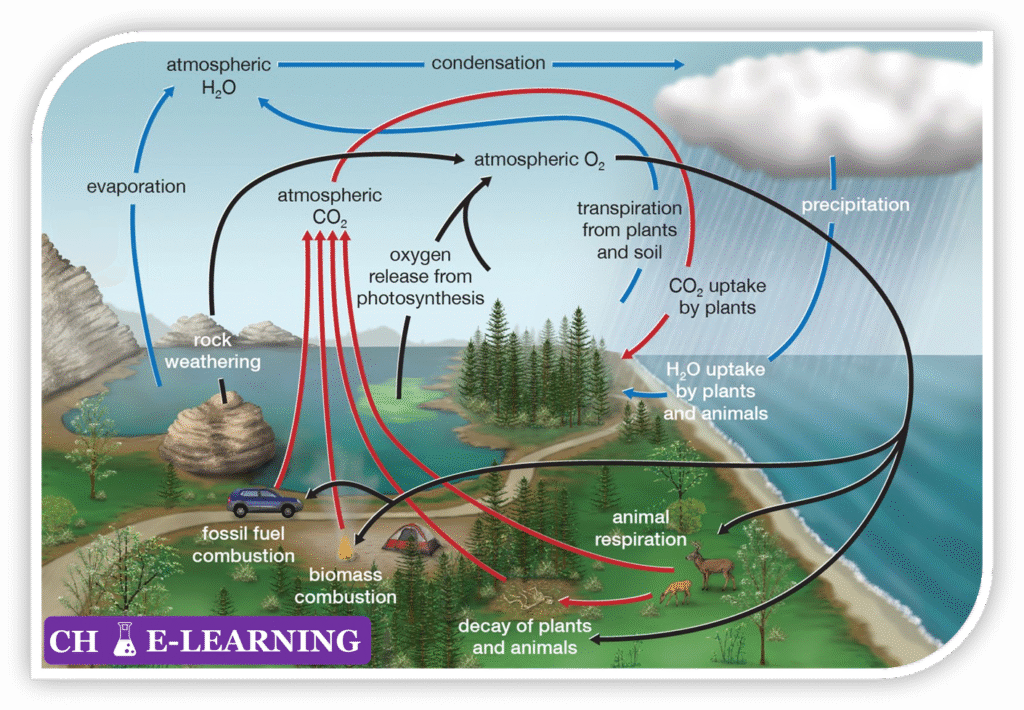
- Photosynthesis: Green plants, algae, and cyanobacteria use sunlight to convert CO₂ and water into glucose and oxygen: \mathrm{6CO_2+6H_2O\rightarrow C_6H_{12}O_6+6O_2}
- This process releases oxygen into the atmosphere and hydrosphere, serving as Earth’s primary source of free oxygen.
- Marine phytoplankton generate approximately 50% of Earth’s oxygen, while land plants contribute the remainder.
- Cellular Respiration: Animals, plants, and microorganisms consume oxygen to break down glucose, releasing CO₂, water, and energy: \mathrm{C_6H_{12}O_6+6O_2\rightarrow6CO_2+6H_2O+ATP}
- Respiration maintains the balance of oxygen and carbon dioxide in ecosystems.
- Decomposition: Fungi and bacteria decompose dead organic matter, consuming oxygen and releasing CO₂, water, and nutrients back into the environment. \mathrm{C_6H_{12}O_6+6O_2\rightarrow6CO_2+6H_2O+nutrients}
- Oceanic Exchange: Ocean-atmosphere gas exchange involves multiple processes:
- Physical Processes:
- Dissolution: Oxygen dissolves in ocean water from the atmosphere, depending on temperature, salinity, and pressure.
- Outgassing: Dissolved oxygen returns to the atmosphere through wave action and diffusion.
- Biological Processes:
- Photosynthetic production: Phytoplankton contribute approximately 50% of global oxygen production.
- Respiratory consumption: Marine organisms consume dissolved oxygen for cellular metabolism
- Photolysis and Ozone Formation: It occurs when high-energy solar radiation breaks apart oxygen molecules in the atmosphere. \mathrm{O_2\xrightarrow{UV\left(\lambda<240\;nm\right)}2O} \mathrm{O_2+O\rightarrow O_3} \mathrm{O_3\xrightarrow{UV\left(240<\lambda<310\;nm\right)}O_2+O}
- This cycle converts harmful UV radiation into heat while maintaining ozone layer stability.
- Geological Processes: Weathering of rocks and oxidation of minerals (e.g., rusting of iron) consume oxygen.
- Volcanic activity and tectonic processes release oxygen-containing compounds back into the atmosphere and oceans.
- High-temperature volcanic processes may consume atmospheric oxygen through mineral oxidation reactions.
Carbon Cycle::
The carbon cycle refers to the continuous movement of carbon among the atmosphere, hydrosphere, biosphere, and lithosphere.
- Carbon is the backbone of all organic molecules such as proteins, carbohydrates, lipids, and nucleic acids (DNA & RNA) and plays a central role in sustaining life, regulating climate, and maintaining ecological balance.
Reservoir of Carbon:::
- Lithosphere: The largest reservoir, storing carbon in sedimentary rocks (limestone, dolomite) and fossil fuels (coal, petroleum, and natural gas).
- This is a long-term storage pool with a very slow exchange rate; most of the Earth’s carbon remains locked here for millions of years.
- Oceans (Hydrosphere): Oceans hold the largest active reservoir of carbon (~93% of Earth’s active carbon), mainly as dissolved inorganic carbon (carbonates CO₃²⁻, bicarbonates HCO₃⁻, dissolved CO₂).
- Carbonates and bicarbonates readily exchange with atmospheric CO₂, regulating marine and global carbon balance.
- Atmosphere: Contains ~750–850 gigatons of carbon, mostly as CO₂ (~0.04% by volume) and smaller amounts as CH₄ (methane), CO (carbon monoxide), and other trace gases.
- Though relatively small compared to oceans and lithosphere, it plays a crucial role in climate regulation.
- Biosphere: Carbon is stored in living organisms as organic molecules (carbohydrates, lipids, proteins, nucleic acids).
- Dead organisms and waste products return carbon to the soil and atmosphere through decomposition.
Forms of Carbon:::
- Inorganic Carbon: CO₂, carbonates (CO₃²⁻), bicarbonates (HCO₃⁻) are found in the atmosphere, oceans, and rocks.
- Organic Carbon: Carbohydrates, fats, proteins, nucleic acids, found in living and dead organisms.
- Gaseous Forms: Carbon dioxide (CO₂), methane (CH₄), carbon monoxide (CO).
- Fossil Carbon: Stored in coal, petroleum, and natural gas.
Processes of Carbon Circulation:::
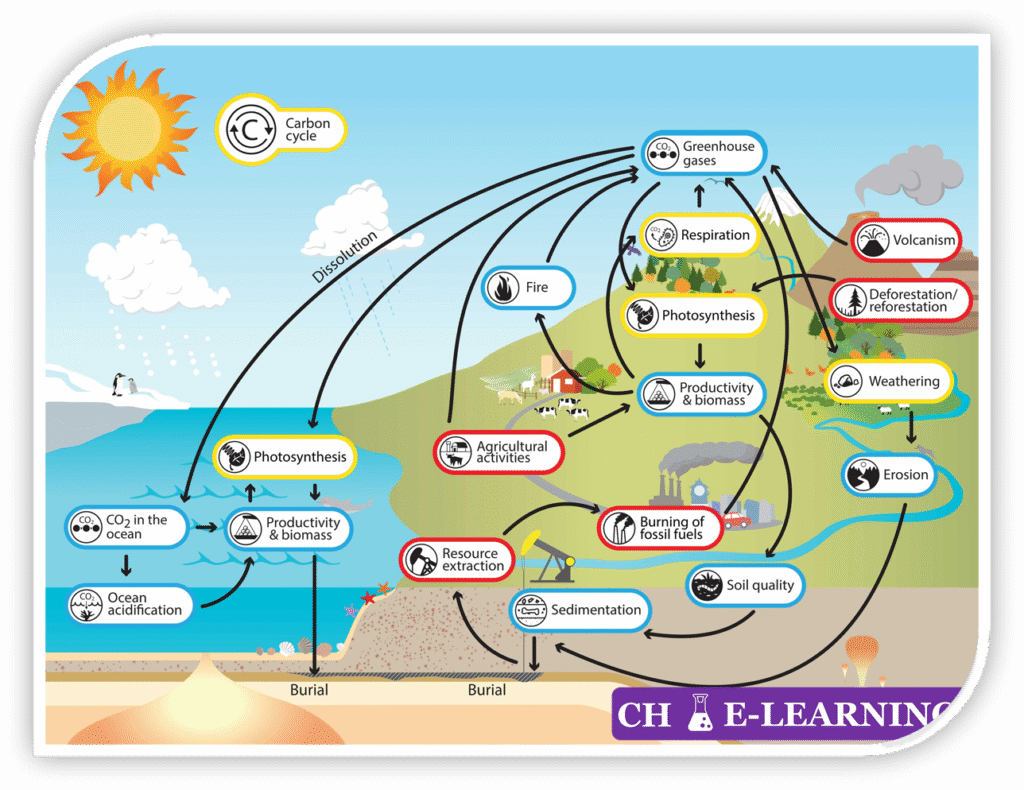
- Photosynthesis: Plants, algae, and cyanobacteria fix atmospheric CO₂ using sunlight to produce glucose and oxygen: \mathrm{6CO_2+6H_2O\rightarrow C_6H_{12}O_6+6O_2}
- This is a process of reduction of CO2 to organic carbon (C6H12O6), introducing carbon into the biosphere and forming the base of food chains.
- Respiration: Plants, animals, and microorganisms oxidize glucose to release energy, producing CO₂ and water: \mathrm{C_6H_{12}O_6+6O_2\rightarrow6CO_2+6H_2O+ATP}
- Decomposition: Fungi and bacteria break down dead organic matter and waste, releasing CO₂, CH₄ (under anaerobic conditions), and nutrients back to the environment.
- Aerobic decomposition \mathrm{C_6H_{12}O_6+6O_2\rightarrow6CO_2+6H_2O}
- Anaerobic decomposition \mathrm{C_6H_{12}O_6\rightarrow3CO_2+3CH_4}
- Oceanic Processes:
- Dissolution: Oceans absorb atmospheric CO₂, where it reacts to form carbonic acid (H₂CO₃), bicarbonate, and carbonate ions. \mathrm{CO_2+H_2O\leftrightarrow H_2CO_3\leftrightarrow HCO_3^-\leftrightarrow CO_3^{2-}+2H^+ }
- Carbonate Formation: Marine organisms use carbonates to build shells and skeletons (CaCO3), which later accumulate as sediments. \mathrm{Ca^{2+}+CO_3^{2-}\rightarrow CaCO_3}
- Biological Pump: Marine photosynthesis converts dissolved CO2 to organic matter, which can sink to deeper ocean layers, forming marine sediments.
- Combustion: The burning of fossil fuels and biomass releases large amounts of CO₂ into the atmosphere. \mathrm{CH_4+2O_2\rightarrow CO_2+2H_2O+heat}
- Human-induced combustion is a major driver of global warming and climate change.
- Geological Processes: Volcanic eruptions release CO₂ stored in Earth’s interior.
- Weathering of silicate rocks consumes atmospheric CO₂ and eventually forms carbonates. \mathrm{CaSiO_3+CO_2\rightarrow CaCO_3+SiO_2}
- Tectonic activity recycles carbon between Earth’s crust and atmosphere.
Nitrogen Cycle::
The nitrogen cycle is the continuous circulation of nitrogen among the atmosphere, hydrosphere, biosphere, and lithosphere.
- Nitrogen is an essential element required for the synthesis of amino acids, proteins, nucleic acids (DNA, RNA), and other essential biomolecules for all living organisms.
Reservoir of Nitrogen:::
- Atmosphere: The largest reservoir, containing ~78% nitrogen gas (N₂). Due to its strong triple bond (N≡N), it is chemically inert and unavailable directly to most organisms.
- Lithosphere: Nitrogen is stored in sedimentary rocks, soil minerals, and deposits such as sodium nitrate (NaNO₃) and potassium nitrate (KNO₃). Additionally, organic nitrogen also resides in fossil fuels and soil organic matter.
- Hydrosphere: Dissolved nitrogen compounds (nitrates NO3-, nitrites NO2-, ammonium NH4+) in oceans, river and lakes.
- Biosphere: Present in living organism as part of proteins, enzymes, nucleic acids, chlorophyll, and other nitrogen-containing compounds.
Forms of Nitrogen:::
- Inorganic nitrogen: Molecular nitrogen (N₂), ammonia (NH₃), ammonium ions (NH₄⁺), nitrite ions (NO₂⁻), nitrate ions (NO₃⁻).
- Organic nitrogen: Nitrogen bound in amino acids, proteins, nucleic acids, urea, and uric acid.
- Nitrogen Oxides: Gaseous forms such as nitric oxide (NO), nitrogen dioxide (NO₂), and nitrous oxide (N₂O).
Processes of Nitrogen Circulation:::
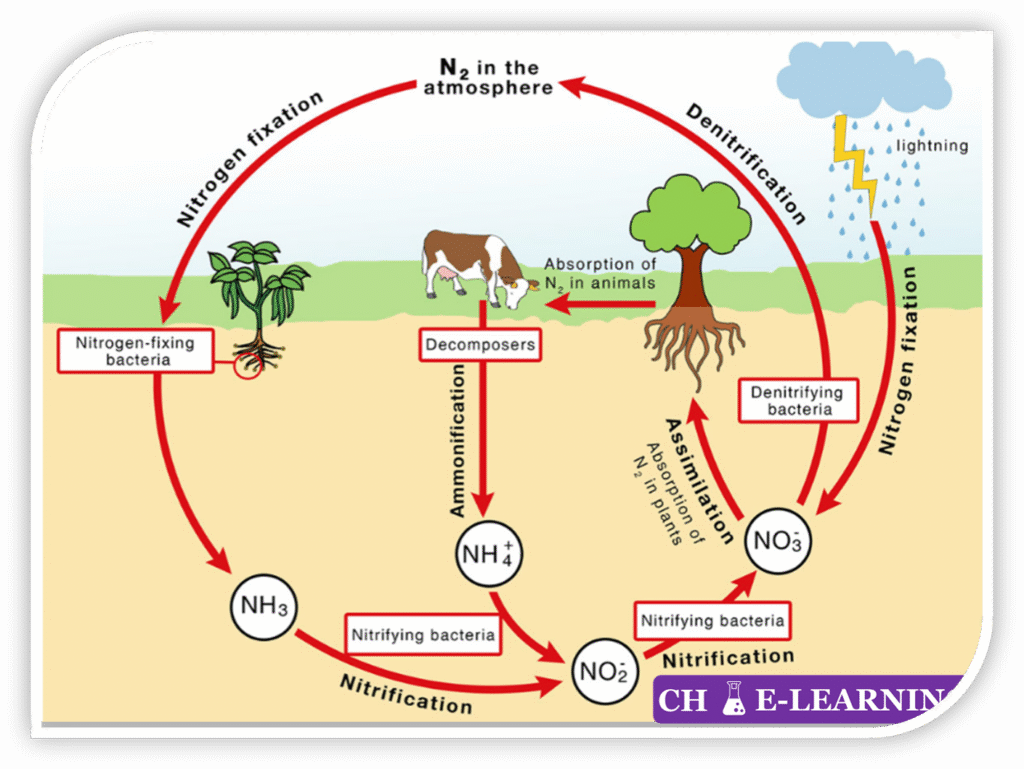
- Nitrogen fixation (N₂ → NH₃/NH₄⁺): The conversion of inert atmospheric nitrogen gas (N₂) into biologically usable forms (ammonia NH₃ & ammonium NH₄⁺) via three mechanisms:
- Biological Fixation: Carried out by nitrogen-fixing bacteria (symbiotic and free-living bacteria). These microbes use the enzyme nitrogenase to reduce N₂ into ammonia under aerobic or anaerobic conditions. \mathrm{N_2+8H^++8e^-\xrightarrow{Nitrogenase}2NH_3+H_2}
- Atmospheric Fixation: Lightning provides sufficient energy to break strong nitrogen bonds, forming nitrogen oxides (NO, NO₂), which dissolve in rainwater to produce nitrates that enrich the soil. \mathrm{N_2+O_2\rightarrow2NO} \mathrm{2NO+O_2\rightarrow2NO_2} \mathrm{3NO_2+H_2O\rightarrow2HNO_3+NO}
- Overall, these steps convert N₂ into nitric acid (HNO₃) in rainwater, which dissociates to nitrate (NO₃⁻) in soil: \mathrm{HNO_3\rightarrow H^++NO_3^- }
- Industrial Fixation (Haber–Bosch process): A synthetic process in which N2 & H2 gases are combined under high temperature (~450 °C) and pressure (200–400 atm) with an iron catalyst to form ammonia, primarily for fertilizers. \mathrm{N_2+3H_2\xrightarrow{Fe\;catalyst}2NH_3}
- Nitrification (NH₃ → NO₂⁻ → NO₃⁻): An aerobic microbial process in which nitrifying bacteria oxidize ammonia into nitrites and then nitrates: \mathrm{NH_3+\frac32O_2\xrightarrow{Nitrosomonas}NO_2^-+H^++H_2O} \mathrm{NO_2^-+\frac12O_2\xrightarrow{Nitrobacter}NO_3^- }
- Plants primarily absorb nitrate (NO₃⁻) as their nitrogen source.
- Assimilation (NH₄⁺/NO₃⁻ → Organic N): Plants and microorganisms assimilate inorganic nitrogen (NH₄⁺ and NO₃⁻) into organic molecules such as amino acids, proteins, DNA, and RNA. \mathrm{NO_3^-\rightarrow NO_2^-\rightarrow NH_4^+\rightarrow Aminoacids\rightarrow Proteins/DNA/RNA }
- Animals obtain nitrogen by consuming plants or other animals, incorporating the nitrogen into their own biomolecules.
- Ammonification (Mineralization) (Organic N → NH₃/NH₄⁺): Decomposes of organic nitrogen compounds from dead organisms and waste into ammonia or ammonium ions, by decomposer bacteria and fungi. \mathrm{Organic\;N\;(proteins,urea,nuclei\;acids)\xrightarrow{bacteria,fungi}NH_3+other\;products} \mathrm{R-NH_2+H_2O\rightarrow NH_3+ROH}
- Denitrification (NO₃⁻ → N₂): Under anaerobic conditions, denitrifying bacteria (e.g., Pseudomonas, Clostridium) reduce nitrates (NO₃⁻) back to gaseous nitrogen (N₂). \mathrm{NO_3^-\rightarrow NO_2^-\rightarrow N_2\uparrow}
- This releases nitrogen into the atmosphere, thereby completing the nitrogen cycle.
Sedimentary Cycle:
Sedimentary cycles are slow biogeochemical cycles in which nutrients such as phosphorus, sulfur, calcium, magnesium, sodium, and iron circulate mainly between rocks, soils, water bodies, and living organisms.
- The Earth’s crust, particularly soils and sediments, serves as the primary reservoir.
- Weathering and erosion of rocks release these elements into soil and water.
- Unlike gaseous cycles, sedimentary cycles operate mostly as one-way flows.
- As a result, ecosystems face a continual risk of shortfall or depletion of these essential nutrients.

Sulphur Cycle::
The sulfur cycle is the biogeochemical circulation of sulfur among the lithosphere, hydrosphere, atmosphere, and biosphere.
- Sulfur is essential for life, particularly as a constituent of certain amino acids (cysteine, methionine), vitamins and enzymes.
- Unlike the relatively fast gaseous cycles (e.g., nitrogen and carbon), the sulfur cycle involves slower sedimentary processes, where large reservoirs of sulfur are locked in rocks and sediments.
Reservoirs of Sulfur:::
- Lithosphere (Primary Long-term Reservoir): The largest sulfur reservoir is found in rocks and minerals:
- Sulfide (S²⁻) minerals: Pyrite (FeS₂).
- Sulfate (SO₄²⁻) minerals: Gypsum (CaSO₄·2H₂O), anhydrite (CaSO₄).
- Evaporite deposits: Sodium sulfate (Na₂SO₄), potassium sulfate (K₂SO₄).
- Fossil fuels: Coal and petroleum contain organic sulfur compounds.
- Hydrosphere (Largest Active Reservoir): Ocean water contains ~28 mM dissolved sulfate (~1.3 × 10²¹ g globally).
- Dissolved forms: Sulfate ions (SO₄²⁻)
- Anaerobic marine sediments contain hydrogen sulfide (H₂S) and elemental sulfur.
- Atmosphere (Smaller, rapidly cycling reservoir): Contains comparatively small but active reservoirs of sulfur gases:
- Sulfur dioxide (SO₂): Released from volcanic eruptions, fossil fuel combustion, and biomass burning.
- Hydrogen sulfide (H₂S): Emitted from anaerobic microbial processes and volcanic eruptions.
- Dimethyl sulfide (CH₃)₂S (DMS): Produced biologically in oceans; important in cloud formation and climate regulation.
- Biosphere: Sulfur occurs in organic molecules (proteins, enzymes) and microbial biomass.
- Organic sulfur in proteins, amino acids, vitamins, and enzymes.
- Approximately 1-2% of living matter contains sulfur compounds.
Forms of Sulfur:::
- Gaseous: Sulfur dioxide (SO₂), Hydrogen sulfide (H₂S), sulfur trioxide (SO3), DMS
- Oxidized: Sulfate (SO₄²⁻), sulfuric acid (H₂SO₄).
- Reduced/Inorganic: Elemental sulfur (S⁰), sulfides (FeS₂), thiosulfate (S₂O₃²⁻), sulfite SO₃²⁻).
- Organic Sulphur: Found in amino acids, proteins, and other biomolecules.
Processes of Sulphur Circulation:::
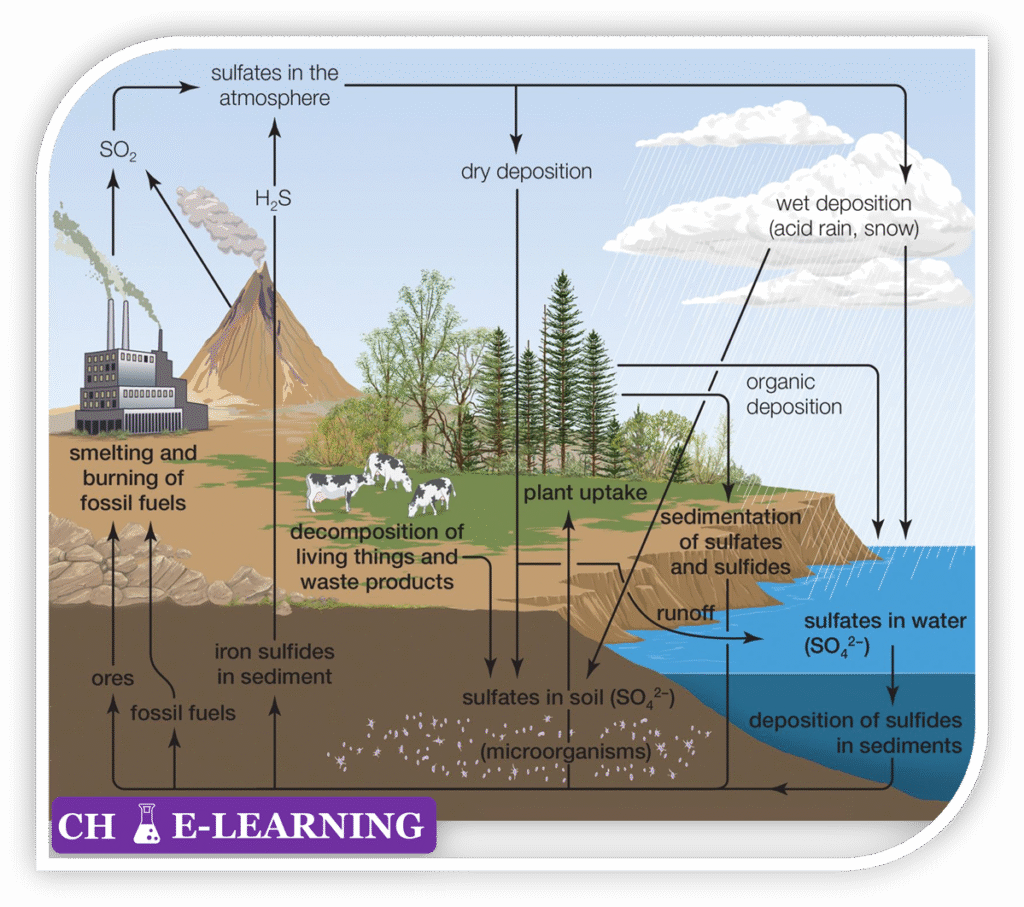
- Weathering (Lithosphere → Soil/Water): Weathering of rocks releases sulfur compounds (sulfate ions) into soil and water systems, making them available for biological uptake. \mathrm{CaSO_4.2H_2O\rightarrow Ca^{2+}+SO_4^{2-}+2H_2O} \mathrm{FeS_2+\frac{15}4O_2+{\textstyle\frac72}H_2O\rightarrow Fe{(OH)}_3+2SO_4^{2-}+4H^+ }
- Assimilation (SO₄²⁻ → Organic S in Plants/Microbes → Animals via food chain): Plants and microbes absorb sulfate ions (SO₄²⁻) from soil or water.
- They convert it into organic sulfur compounds (e.g., amino acids, proteins).
- Animals acquire sulfur by consuming plants or other organisms.
- Decomposition and Mineralization (Organic S → H₂S / SO₄²⁻): When plants and animals die, decomposers (bacteria and fungi) break down organic sulfur compounds. \mathrm{Protein‐S\rightarrow H_2S+NH_3}
- This releases H₂S or sometimes sulfate back into the environment.
- Microbial Transformations
- Sulfate Reduction (Anaerobic SO₄²⁻ → H₂S): In oxygen-poor environments (sediments, swamps), sulfate-reducing bacteria (desulfovibrio, desulfotomaculum) use sulfate as an electron acceptor: \mathrm{SO_4^{2-}+8e^-+10H^+\rightarrow H_2S+4H_2O}
- Sulfur Oxidation (Aerobic H₂S → S⁰ → SO₄²⁻): Sulfur-oxidizing bacteria (thiobacillus, beggiatoa, acidithiobacillus) convert reduced sulfur back to sulfate: \mathrm{H_2S+\frac12O_2\rightarrow S^0+H_2O} \mathrm{S^0+\frac32O_2+H_2O\rightarrow SO_4^{2-}+2H^+ }
- Chemolithotrophs oxidize elemental sulfur (S⁰) to sulfate, making it bioavailable again.
- Atmospheric Processes:
- Volcanic emissions: Release SO₂ and H₂S into the atmosphere. \mathrm{H_2S+\frac32O_2\rightarrow SO_2+H_2O}
- Atmospheric Oxidation: \mathrm{2SO_2+O_2\rightarrow2SO_3} \mathrm{SO_3+H_2O\rightarrow H_2SO_4}
- Oxidation of DMS: \mathrm{(CH_3)_2S+3O_2\rightarrow SO_2+2CH_2O+H_2O}
- Acid precipitation: Sulfuric acid returns to Earth as acid rain, altering soil and water chemistry. \mathrm{H_2SO_4\rightarrow2H^++SO_4^{2-} }
Phosphorus Cycle::
The phosphorus cycle describes the biogeochemical movement of phosphorus through the lithosphere, hydrosphere, and biosphere.
- Unlike carbon and nitrogen cycles, phosphorus has negligible presence in the atmosphere and primarily moves through solid, aqueous, and biological phases.
- Phosphorus (P) is a vital nutrient for all living organisms, forming key components of DNA, RNA, ATP, and phospholipid membranes.
- In humans, ~80% of phosphorus resides in bones and teeth as hydroxyapatite [Ca₅(PO₄)₃OH].
Reservoirs of Phosphorus:::
- Lithosphere (Primary Long-term Reservoir): Stored in igneous and sedimentary rocks, mainly as apatite minerals (Ca₅(PO₄)₃OH, Ca₅(PO₄)₃F, Ca₅(PO₄)₃Cl) and other insoluble phosphate salts.
- Weathering and erosion of these rocks gradually release phosphate (PO₄³⁻) into soils and the aquatic system.
- Soils and freshwater sediments act as short-term, biologically active pools of phosphorus.
- Hydrosphere: Dissolved inorganic phosphate (PO₄³⁻) is the main form in rivers, lakes, and oceans.
- Rivers transport dissolved and particulate phosphorus to marine systems.
- Most phosphorus is deposited in near-shore sediments, while only a small fraction reaches the open ocean.
- Biosphere: Plants assimilate phosphate into organic molecules (nucleic acids, ATP, phospholipids).
- Animals obtain phosphorus by consuming plants or other animals, incorporating it into bone, teeth, and tissues.
- Decomposition and mineralization recycle phosphorus back to soil and water.
Forms of Phosphorus::
- Inorganic phosphate (PO₄³⁻): Orthophosphate ions (PO₄³⁻) in soils and water.
- Organic phosphorus: Found in biomass (nucleic acids, lipids, detritus, phospholipids, ATP).
- Particulate Phosphorus: Adsorbed to soil minerals (iron phosphate FePO₄, aluminum phosphate AlPO₄) or in detritus (organic matter made up of the decomposing remains of organisms and plants).
Processes of Phosphorus Circulation:::

- Weathering & Erosion (Rocks → Soil/Water): Phosphate minerals are gradually broken down by weathering and erosion, liberating PO₄³⁻ into soils and freshwater systems. \mathrm{Ca_5(PO_4)_3OH\rightarrow5Ca^{2+}+3PO_4^{3-}+OH^- }
- Assimilation (Soil/Water → Organisms): Plants and microbes absorb PO₄³⁻ from soil and water and convert it into organic phosphorus.
- Animals acquire phosphorus through the consumption of plant or animal biomass.
- Decomposition & Mineralization (Organisms → Soil/Water): Decomposer bacteria and fungi break down the organic phosphorus back to PO₄³⁻, replenishing soil and water phosphate. \mathrm{R‐PO_4\rightarrow PO_4^{3-}}
- Runoff & Sedimentation (Soil → Aquatic Systems → Sediments): Excess phosphate leaches into waterways, where it precipitates with calcium or adsorbs to particles, settling into aquatic sediments. \mathrm{3Ca^{2+}+2PO_4^{3-}\rightarrow Ca_3(PO_4)_2\downarrow}
- Geological Uplift (Sediments → Land): Over geologic timescales, tectonic processes expose phosphate-rich marine sediments to surface weathering, completing the long-term cycle.
- Guano Deposits: Seabird droppings (guano) accumulate as phosphorus-rich deposits (mainly calcium phosphate (Ca₃(PO₄)₂) and uric acid derivatives) on islands and coastal cliffs, historically exploited as high-grade fertilizer.
Human Impacts
- Phosphate Mining & Fertilizer Use: Greatly accelerates phosphorus mobilization from rock to soil and water.
- Eutrophication: Runoff from agriculture elevates phosphorus in water bodies, triggering algal blooms, oxygen depletion, and ecosystem degradation.
