Solids have a strongest intermolecular force than liquids and gases. Then, why the heat of vaporization is more than the heat of fusion?
In this post, you will learn why the heat of vaporization is more than the heat of fusion despite solids having the strongest intermolecular force as compared to liquid & gas.
Let us first examine the transitions from solid to liquid and liquid to gas.
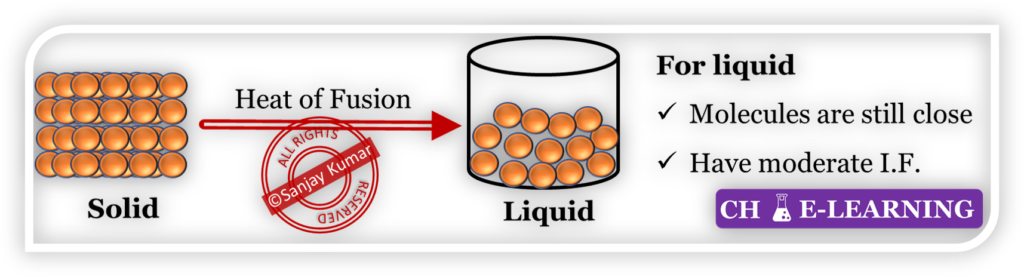
Solid to Liquid Phase Transition:
- In the case of solid, molecules are very closely packed and have the strongest intermolecular forces (I.F.).
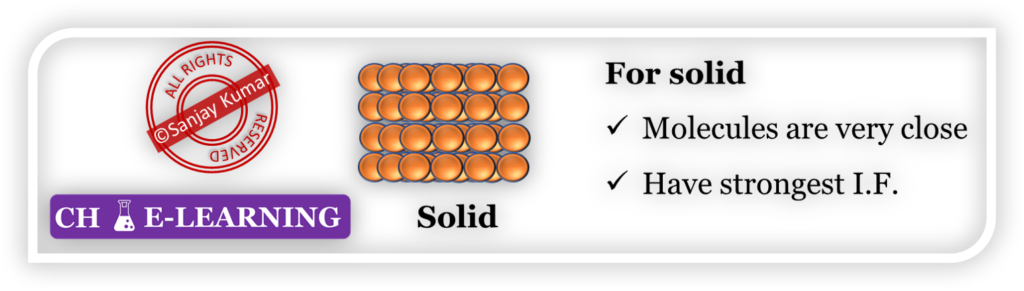
- For changing the state, energy is required to overcome these intermolecular forces.
- During the transition from solid to liquid, the kinetic energy of molecules increases which allows them to move apart and flow freely.
- As a result, intermolecular forces decrease in liquid due to increased distance between molecules. However, intermolecular forces still exist in the liquid state.
Liquid to Gas Phase Transition:
During the transition from liquid to gas,
- The kinetic energy of molecules becomes very high due to which molecules move far apart.
- As a result, negligible intermolecular forces exist among gas molecules.

Therefore, the energy required to completely separate the molecules from each other is much more than the energy required just to reduce the separation among molecules.
Conclusions:

- A change of state from liquid to gas requires higher energy as compared to a change of state from solid to liquid.
- Because during the transition (Solid ↔ Liquid ↔ Gas) only the distance among molecules changes, molecules themselves do not break out.
- For example, water doesn’t break into hydrogen and oxygen during a change of state.
- For water, \mathrm{\triangle H_{fusion}=6\;kJ/mol} and \mathrm{\triangle H_{vaporization}=40.7\;kJ/mol}
\mathrm{\triangle H_{vaporization}>\triangle H_{fusion}}

Thnx
The breadth of knowledge compiled on this website is astounding. Every article is a well-crafted masterpiece brimming with insights. I’m grateful to have discovered such a rich educational resource. You’ve gained a lifelong fan!
Grateful for your kind words! Happy learning.
Usually I do not read article on blogs however I would like to say that this writeup very compelled me to take a look at and do so Your writing taste has been amazed me Thanks quite nice post
Your blog is a shining example of excellence in content creation. I’m continually impressed by the depth of your knowledge and the clarity of your writing. Thank you for all that you do.